Mobilization of Transplanted Bone Marrow Mesenchymal Stem Cells by Erythropoietin Facilitates the Reconstruction of Segmental Bone Defect
- PMID: 31065275
- PMCID: PMC6466852
- DOI: 10.1155/2019/5750967
Mobilization of Transplanted Bone Marrow Mesenchymal Stem Cells by Erythropoietin Facilitates the Reconstruction of Segmental Bone Defect
Abstract
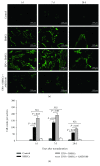
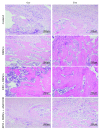
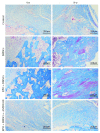
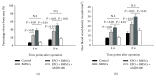
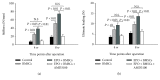
Reconstruction of segmental bone defects poses a tremendous challenge for both orthopedic clinicians and scientists, since bone rehabilitation is requisite substantially and may be beyond the capacity of self-healing. Bone marrow mesenchymal stem cells (BMSCs) have been identified as an optimal progenitor cell source to facilitate bone repair since they have a higher ability for proliferation and are more easily accessible than mature osteoblastic cells. In spite of the potential of BMSCs in regeneration medicine, particularly for bone reconstruction, noteworthy limitations still remain in previous application of BMSCs, including the amount of cells that could be recruited, the compromised bone migration of grafted cells, reduced proliferation and osteoblastic differentiation ability, and likely tumorigenesis. Our current work demonstrates that BMSCs transplanted through the caudal vein can be mobilized by erythropoietin (EPO) to the bone defect area and participate in regeneration of new bone. Based on the histological analysis and micro-CT findings of this study, EPO can dramatically promote the effects on the osteogenesis and angiogenesis efficiency of BMSCs in vivo. Animals that underwent EPO+BMSC administration demonstrated a remarkable increase in new bone formation, tissue structure organization, new vessel density, callus formation, and bone mineral density (BMD) compared with the BMSCs alone and control groups. At the biomechanical level, we demonstrated that combing transplantation of EPO and BMSCs enhances bone defect reconstruction by increasing the strength of the diaphysis, making it less fragile. Therefore, combination therapy using EPO infusion and BMSC transplantation may be a new therapeutic strategy for the reconstruction of segmental bone defect.
Figures



Similar articles
-
Endothelial progenitor cells improve the therapeutic effect of mesenchymal stem cell sheets on irradiated bone defect repair in a rat model.J Transl Med. 2018 May 22;16(1):137. doi: 10.1186/s12967-018-1517-4. J Transl Med. 2018. PMID: 29788957 Free PMC article.
-
Erythropoietin facilitates the recruitment of bone marrow mesenchymal stem cells to sites of spinal cord injury.Exp Ther Med. 2017 May;13(5):1806-1812. doi: 10.3892/etm.2017.4182. Epub 2017 Mar 6. Exp Ther Med. 2017. PMID: 28565771 Free PMC article.
-
Bone mesenchymal stem cells pretreated with erythropoietin enhance the effect to ameliorate cyclosporine A-induced nephrotoxicity in rats.J Cell Biochem. 2018 Nov;119(10):8220-8232. doi: 10.1002/jcb.26833. Epub 2018 Jun 22. J Cell Biochem. 2018. PMID: 29932236
-
Effect of erythropoietin on the migration of bone marrow-derived mesenchymal stem cells to the acute kidney injury microenvironment.Exp Cell Res. 2013 Aug 1;319(13):2019-2027. doi: 10.1016/j.yexcr.2013.04.008. Epub 2013 Apr 24. Exp Cell Res. 2013. PMID: 23624354
-
Mechanism of Action of Icariin in Bone Marrow Mesenchymal Stem Cells.Stem Cells Int. 2019 Apr 4;2019:5747298. doi: 10.1155/2019/5747298. eCollection 2019. Stem Cells Int. 2019. PMID: 31089330 Free PMC article. Review.
Cited by
-
Concentrated Growth Factors (CGF) Induce Osteogenic Differentiation in Human Bone Marrow Stem Cells.Biology (Basel). 2020 Oct 30;9(11):370. doi: 10.3390/biology9110370. Biology (Basel). 2020. PMID: 33143015 Free PMC article.
-
Repair of segmental bone defect using tissue engineered heterogeneous deproteinized bone doped with lithium.Sci Rep. 2021 Mar 1;11(1):4819. doi: 10.1038/s41598-021-84526-w. Sci Rep. 2021. PMID: 33649409 Free PMC article.
-
The Role of PI3K/AKT and MAPK Signaling Pathways in Erythropoietin Signalization.Int J Mol Sci. 2021 Jul 19;22(14):7682. doi: 10.3390/ijms22147682. Int J Mol Sci. 2021. PMID: 34299300 Free PMC article. Review.
-
Systemic therapy of MSCs in bone regeneration: a systematic review and meta-analysis.Stem Cell Res Ther. 2021 Jul 2;12(1):377. doi: 10.1186/s13287-021-02456-w. Stem Cell Res Ther. 2021. PMID: 34215342 Free PMC article.
-
Research progress on the role and mechanism of magnesium-containing materials in bone repair.Biomater Transl. 2025 Jun 25;6(2):114-126. doi: 10.12336/bmt.24.00038. eCollection 2025. Biomater Transl. 2025. PMID: 40641988 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Research Materials

