Cross-over in the dynamics of polymer confined between two liquids of different viscosity
- PMID: 31065342
- PMCID: PMC6501349
- DOI: 10.1098/rsfs.2018.0074
Cross-over in the dynamics of polymer confined between two liquids of different viscosity
Abstract
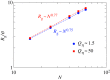
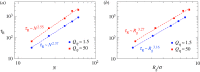
Using molecular dynamics simulations, we analysed the polymer dynamics of chains of different molecular weights entrapped at the interface between two immiscible liquids. We showed that on increasing the viscosity of one of the two liquids the dynamic behaviour of the chain changes from a Zimm-like dynamics typical of dilute polymer solutions to a Rouse-like dynamics where hydrodynamic interactions are screened. We observed that when the polymer is in contact with a high viscosity liquid, the number of solvent molecules close to the polymer beads is reduced and ascribed the screening effect to this reduced number of polymer-solvent contacts. For the longest chain simulated, we calculated the distribution of loop length and compared the results with the theoretical distribution developed for solid/liquid interfaces. We showed that the polymer tends to form loops (although flat against the interface) and that the theory works reasonably well also for liquid/liquid interfaces.
Keywords: Rouse model; Zimm model; free draining; hydrodynamics; polymer dynamics.
Conflict of interest statement
We declare we have no competing interests.
Figures





References
-
- Fleer G, Stuart MC, Scheutjens JM, Cosgrove T, Vincent B. 1993. Polymers at interfaces. Berlin, Germany: Springer Science & Business Media.
-
- Cheung DL, Carbone P. 2013. How stable are amphiphilic dendrimers at the liquid-liquid interface? Soft Matter 9, 6841–6850. ( 10.1039/C2SM27246F) - DOI
Associated data
LinkOut - more resources
Full Text Sources
