Multi-wavelength, en-face photoacoustic microscopy and optical coherence tomography imaging for early and selective detection of laser induced retinal vein occlusion
- PMID: 31065403
- PMCID: PMC6491003
- DOI: 10.1364/BOE.9.005915
Multi-wavelength, en-face photoacoustic microscopy and optical coherence tomography imaging for early and selective detection of laser induced retinal vein occlusion
Abstract
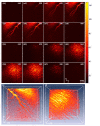
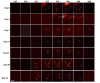
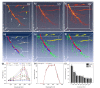
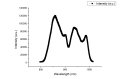
Multi-wavelength en face photoacoustic microscopy (PAM) was integrated with a spectral domain optical coherence tomography (SD-OCT) to evaluate optical properties of retinal vein occlusion (RVO) and retinal neovascularization (RNV) in living rabbits. The multi-wavelength PAM of the RVO and RNV were performed at several wavelengths ranging from 510 to 600 nm. Rose Bengal-induced RVO and RNV were performed and evaluated on eight rabbits using color fundus photography, fluorescein angiography, OCT, and spectroscopic en face PAM. In vivo experiment demonstrates that the spectral variation of photoacoustic response was achieved. The location and the treatment margins of the occluded vasculature as well as the morphology of individual RNV were obtained with high contrast at a laser energy of 80 nJ, which was only half of the American National Standards Institute safety limit. In addition, dynamic changes in the retinal morphology and retinal neovascularization were administered using PA spectroscopy at numerous time points: 0, 3, 7, 14, 21, 28, and 35 days after photocoagulation. The proposed multi-wavelength spectroscopic PAM imaging may provide a potential imaging platform to differentiate occluded retinal vasculature and to improve characterization of microvasculature in a safe and efficient manner.
Conflict of interest statement
The authors declare that there are no conflicts of interest related to this article
Figures










References
-
- Rogers S., McIntosh R. L., Cheung N., Lim L., Wang J. J., Mitchell P., Kowalski J. W., Nguyen H., Wong T. Y., “The Prevalence of Retinal Vein Occlusion: Pooled Data from Population Studies from the United States, Europe, Asia, and Australia,” Ophthalmology 117(2), 313 (2010). 10.1016/j.ophtha.2009.07.017 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
