Computational multifocal microscopy
- PMID: 31065444
- PMCID: PMC6491004
- DOI: 10.1364/BOE.9.006477
Computational multifocal microscopy
Abstract
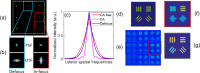
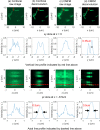
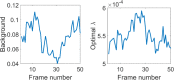
Despite recent advances, high performance single-shot 3D microscopy remains an elusive task. By introducing designed diffractive optical elements (DOEs), one is capable of converting a microscope into a 3D "kaleidoscope," in which case the snapshot image consists of an array of tiles and each tile focuses on different depths. However, the acquired multifocal microscopic (MFM) image suffers from multiple sources of degradation, which prevents MFM from further applications. We propose a unifying computational framework which simplifies the imaging system and achieves 3D reconstruction via computation. Our optical configuration omits optical elements for correcting chromatic aberrations and redesigns the multifocal grating to enlarge the tracking area. Our proposed setup features only one single grating in addition to a regular microscope. The aberration correction, along with Poisson and background denoising, are incorporated in our deconvolution-based fully-automated algorithm, which requires no empirical parameter-tuning. In experiments, we achieve spatial resolutions of 0.35um (lateral) and 0.5um (axial), which are comparable to the resolution that can be achieved with confocal deconvolution microscopy. We demonstrate a 3D video of moving bacteria recorded at 25 frames per second using our proposed computational multifocal microscopy technique.
Conflict of interest statement
The authors declare that there are no conflicts of interest related to this article.
Figures









References
-
- Abrahamsson S., Ilic R., Wisniewski J., Mehl B., Yu L., Chen L., Davanco M., Oudjedi L., Fiche J.-B., Hajj B., Jin X., Pulupa J., Cho C., Mir M., Beheiry M. E., Darzacq X., Nollmann M., Dahan M., Wu C., Lionnet T., Liddle J. A., Bargmann C. I., “Multifocus microscopy with precise color multi-phase diffractive optics applied in functional neuronal imaging,” Biomed. Opt. Express 7, 855–869 (2016).10.1364/BOE.7.000855 - DOI - PMC - PubMed
-
- Abrahamsson S., Chen B. H. J., Stallinga S., Katsov A. Y., Wisniewski J., Mizuguchi G., Soule P., Mueller F., Darzacq C. D., Darzacq X., Wu C., Bargmann C. I., Agard D. A., Dahan M., Gustafsson M. G. L., “Fast multicolor 3d imaging using aberration-corrected multifocus microscopy,” Nat. methods 10, 60–63 (2013).10.1038/nmeth.2277 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
