Transcranial magnetic stimulation in anxiety and trauma-related disorders: A systematic review and meta-analysis
- PMID: 31066227
- PMCID: PMC6576151
- DOI: 10.1002/brb3.1284
Transcranial magnetic stimulation in anxiety and trauma-related disorders: A systematic review and meta-analysis
Abstract
Background: Transcranial magnetic stimulation (TMS) has been evaluated as an effective treatment option for patients with major depressive disorder. However, there are limited studies that have evaluated the efficacy of TMS for other neuropsychiatric disorders such as anxiety and trauma-related disorders. We reviewed the literature that has evaluated TMS as a treatment for anxiety and trauma-related disorders.
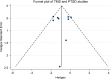
Methods: We searched for articles published up to December 2017 in Embase, Medline, and ISI Web of Science databases, following the Preferred Items for Reporting of Systematic Reviews and Meta-Analyses (PRISMA) statement. Articles (n = 520) evaluating TMS in anxiety and trauma-related disorders were screened and a small subset of these that met the eligibility criteria (n = 17) were included in the systematic review, of which nine evaluated TMS in posttraumatic stress disorder (PTSD), four in generalized anxiety disorder (GAD), two in specific phobia (SP), and two in panic disorder (PD). The meta-analysis was performed with PTSD and GAD since PD and SP had an insufficient number of studies and sample sizes.
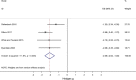
Results: Among anxiety and trauma-related disorders, TMS has been most widely studied as a treatment for PTSD. TMS demonstrated large overall treatment effect for both PTSD (ES = -0.88, 95% CI: -1.42, -0.34) and GAD (ES = -2.06, 95% CI: -2.64, -1.48), including applying high frequency over the right dorsolateral prefrontal cortex. Since few studies have evaluated TMS for SP and PD, few conclusions can be drawn.
Conclusions: Our meta-analysis suggests that TMS may be an effective treatment for GAD and PTSD.
Keywords: anxiety disorders; meta-analysis; posttraumatic stress disorder; systematic review; theta burst; transcranial magnetic stimulation.
© 2019 The Authors. Brain and Behavior published by Wiley Periodicals, Inc.
Conflict of interest statement
AKG receives research support from NIMH
Figures



References
-
- Association, A. P. (2000). Diagnostic and statistical manual of mental disorders (4th text rev. ed.). Washington, DC: Author.
-
- Ballenger, J. C. , Davidson, J. R. , Lecrubier, Y. , Nutt, D. J. , Marshall, R. D. , Nemeroff, C. B. , … Yehuda, R. (2004). Consensus statement update on posttraumatic stress disorder from the international consensus group on depression and anxiety. The Journal of Clinical Psychiatry, 65(Suppl 1), 55–62. - PubMed
-
- Boggio, P. S. , Rocha, M. , Oliveira, M. O. , Fecteau, S. , Cohen, R. B. , Campanhã, C. , … Fregni, F. (2010). Noninvasive brain stimulation with high‐frequency and low‐intensity repetitive transcranial magnetic stimulation treatment for posttraumatic stress disorder. The Journal of Clinical Psychiatry, 71(8), 992–999. 10.4088/JCP.08m04638blu - DOI - PMC - PubMed
-
- Boggio, P. S. , Valasek, C. A. , Campanha, C. , Giglio, A. C. , Baptista, N. I. , Lapenta, O. M. , & Fregni, F. (2011). Non‐invasive brain stimulation to assess and modulate neuroplasticity in Alzheimer's disease. Neuropsychological Rehabilitation, 21(5), 703–716. 10.1080/09602011.2011.617943 - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

