Differential posttranslational modification of mitochondrial enzymes corresponds with metabolic suppression during hibernation
- PMID: 31067076
- PMCID: PMC6732429
- DOI: 10.1152/ajpregu.00052.2019
Differential posttranslational modification of mitochondrial enzymes corresponds with metabolic suppression during hibernation
Abstract
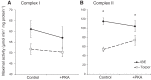
During hibernation, small mammals, including the 13-lined ground squirrel (Ictidomys tridecemlineatus), cycle between two distinct metabolic states: torpor, where metabolic rate is suppressed by >95% and body temperature falls to ~5°C, and interbout euthermia (IBE), where both metabolic rate and body temperature rapidly increase to euthermic levels. Suppression of whole animal metabolism during torpor is paralleled by rapid, reversible suppression of mitochondrial respiration. We hypothesized that these changes in mitochondrial metabolism are regulated by posttranslational modifications to mitochondrial proteins. Differential two-dimensional gel electrophoresis and two-dimensional blue-native PAGE revealed differences in the isoelectric point of several liver mitochondrial proteins between torpor and IBE. Quadrupole time-of-flight LC/MS and matrix-assisted laser desorption/ionization MS identified these as proteins involved in β-oxidation, the tricarboxylic acid cycle, reactive oxygen species detoxification, and the electron transport system (ETS). Immunoblots revealed that subunit 1 of ETS complex IV was acetylated during torpor but not IBE. Phosphoprotein staining revealed significantly greater phosphorylation of succinyl-CoA ligase and the flavoprotein subunit of ETS complex II in IBE than torpor. In addition, the 75-kDa subunit of ETS complex I was 1.5-fold more phosphorylated in torpor. In vitro treatment with alkaline phosphatase increased the maximal activity of complex I from liver mitochondria isolated from torpid, but not IBE, animals. By contrast, phosphatase treatment decreased complex II activity in IBE but not torpor. These findings suggest that the rapid changes in mitochondrial metabolism in hibernators are mediated by posttranslational modifications of key metabolic enzymes, perhaps by intramitochondrial kinases and deacetylases.
Keywords: acetylation; metabolic suppression; mitochondrial metabolism; phosphorylation; succinate dehydrogenase.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures




References
-
- Acín-Pérez R, Carrascoso I, Baixauli F, Roche-Molina M, Latorre-Pellicer A, Fernández-Silva P, Mittelbrunn M, Sanchez-Madrid F, Pérez-Martos A, Lowell CA, Manfredi G, Enríquez JA. ROS-triggered phosphorylation of complex II by Fgr kinase regulates cellular adaptation to fuel use. Cell Metab 19: 1020–1033, 2014. doi:10.1016/j.cmet.2014.04.015. - DOI - PMC - PubMed
-
- Augereau O, Claverol S, Boudes N, Basurko M-J, Bonneu M, Rossignol R, Mazat J-P, Letellier T, Dachary-Prigent J. Identification of tyrosine-phosphorylated proteins of the mitochondrial oxidative phosphorylation machinery. Cell Mol Life Sci 62: 1478–1488, 2005. doi:10.1007/s00018-005-5005-7. - DOI - PMC - PubMed
-
- Brooks SPJ, Storey KB. Mechanisms of glycolytic control during hibernation in the ground squirrel Spermophilus lateralis. J Comp Physiol B 162: 23–28, 1992. doi:10.1007/BF00257932. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

