Local Consolidative Therapy Vs. Maintenance Therapy or Observation for Patients With Oligometastatic Non-Small-Cell Lung Cancer: Long-Term Results of a Multi-Institutional, Phase II, Randomized Study
- PMID: 31067138
- PMCID: PMC6599408
- DOI: 10.1200/JCO.19.00201
Local Consolidative Therapy Vs. Maintenance Therapy or Observation for Patients With Oligometastatic Non-Small-Cell Lung Cancer: Long-Term Results of a Multi-Institutional, Phase II, Randomized Study
Abstract
Purpose: Our previously published findings reported that local consolidative therapy (LCT) with radiotherapy or surgery improved progression-free survival (PFS) and delayed new disease in patients with oligometastatic non-small-cell lung cancer (NSCLC) that did not progress after front-line systemic therapy. Herein, we present the longer-term overall survival (OS) results accompanied by additional secondary end points.
Patients and methods: This multicenter, randomized, phase II trial enrolled patients with stage IV NSCLC, three or fewer metastases, and no progression at 3 or more months after front-line systemic therapy. Patients were randomly assigned (1:1) to maintenance therapy or observation (MT/O) or to LCT to all active disease sites. The primary end point was PFS; secondary end points were OS, toxicity, and the appearance of new lesions. All analyses were two sided, and P values less than .10 were deemed significant.
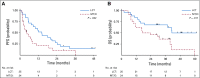
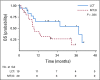
Results: The Data Safety and Monitoring Board recommended early trial closure after 49 patients were randomly assigned because of a significant PFS benefit in the LCT arm. With an updated median follow-up time of 38.8 months (range, 28.3 to 61.4 months), the PFS benefit was durable (median, 14.2 months [95% CI, 7.4 to 23.1 months] with LCT v 4.4 months [95% CI, 2.2 to 8.3 months] with MT/O; P = .022). We also found an OS benefit in the LCT arm (median, 41.2 months [95% CI, 18.9 months to not reached] with LCT v 17.0 months [95% CI, 10.1 to 39.8 months] with MT/O; P = .017). No additional grade 3 or greater toxicities were observed. Survival after progression was longer in the LCT group (37.6 months with LCT v 9.4 months with MT/O; P = .034). Of the 20 patients who experienced progression in the MT/O arm, nine received LCT to all lesions after progression, and the median OS was 17 months (95% CI, 7.8 months to not reached).
Conclusion: In patients with oligometastatic NSCLC that did not progress after front-line systemic therapy, LCT prolonged PFS and OS relative to MT/O.
Figures

Comment in
-
Benefit from LCT in oligometastatic NSCLC.Nat Rev Clin Oncol. 2019 Aug;16(8):466. doi: 10.1038/s41571-019-0233-1. Nat Rev Clin Oncol. 2019. PMID: 31123347 No abstract available.
-
Randomized phase II trial reporting overall survival advantage by adding local consolidative therapy to systemic therapy for oligometastatic non-small cell lung cancer: another step forward on the long road of evidence-based medicine for oligometastatic disease.J Thorac Dis. 2019 Sep;11(Suppl 15):S1869-S1873. doi: 10.21037/jtd.2019.08.123. J Thorac Dis. 2019. PMID: 31632771 Free PMC article. No abstract available.
-
Local consolidative therapy in metastatic non-small cell lung cancer.J Thorac Dis. 2019 Sep;11(Suppl 15):S1909-S1912. doi: 10.21037/jtd.2019.08.93. J Thorac Dis. 2019. PMID: 31632783 Free PMC article. No abstract available.
-
[Local consolidative therapy vs. maintenance therapy or observation for patients with oligometastatic non-small-cell lung cancer: long-term results of a multi-institutional, phase II, randomized study].Strahlenther Onkol. 2019 Dec;195(12):1113-1115. doi: 10.1007/s00066-019-01528-4. Strahlenther Onkol. 2019. PMID: 31637448 German. No abstract available.
-
Is local consolidative therapy adequate for the treatment of oligometastatic non-small cell lung cancer?J Thorac Dis. 2019 Oct;11(10):E154-E157. doi: 10.21037/jtd.2019.09.80. J Thorac Dis. 2019. PMID: 31737327 Free PMC article. No abstract available.
-
Improved clinical outcomes following additional aggressive local consolidative therapy to systemic therapy for oligometastasis from lung cancer: where do we stand between "reality" and "illusion"?J Thorac Dis. 2019 Nov;11(11):E217-E220. doi: 10.21037/jtd.2019.10.18. J Thorac Dis. 2019. PMID: 31903286 Free PMC article. No abstract available.
-
Paradigm shift in local consolidative therapy for oligometastatic non-small cell lung cancer: a meta-analysis.Ann Transl Med. 2019 Dec;7(Suppl 8):S320. doi: 10.21037/atm.2019.09.147. Ann Transl Med. 2019. PMID: 32016038 Free PMC article. No abstract available.
-
Local consolidative therapy for oligometastatic non-small cell lung cancer.J Thorac Dis. 2019 Dec;11(12):5649-5651. doi: 10.21037/jtd.2019.11.19. J Thorac Dis. 2019. PMID: 32030290 Free PMC article. No abstract available.
-
Local consolidative therapy for oligometastatic patients with stage IV non-small cell lung cancer may improve survival, but unanswered questions remain.Transl Lung Cancer Res. 2019 Dec;8(Suppl 4):S407-S411. doi: 10.21037/tlcr.2019.06.07. Transl Lung Cancer Res. 2019. PMID: 32038926 Free PMC article. No abstract available.
-
Surgery for stage IV non-small cell lung cancer?J Thorac Dis. 2020 Apr;12(4):1612-1614. doi: 10.21037/jtd.2020.03.32. J Thorac Dis. 2020. PMID: 32395298 Free PMC article. No abstract available.
References
-
- Weichselbaum RR, Hellman S. Oligometastases revisited. Nat Rev Clin Oncol. 2011;8:378–382. - PubMed
-
- Hellman S, Weichselbaum RR. Oligometastases. J Clin Oncol. 1995;13:8–10. - PubMed
-
- Gomez DR, Blumenschein GR, Jr, Lee JJ, et al. Local consolidative therapy versus maintenance therapy or observation for patients with oligometastatic non–small-cell lung cancer without progression after first-line systemic therapy: A multicentre, randomised, controlled, phase 2 study. Lancet Oncol. 2016;17:1672–1682. - PMC - PubMed
-
- Ost P, Reynders D, Decaestecker K, et al. Surveillance or metastasis-directed therapy for oligometastatic prostate cancer recurrence: A prospective, randomized, multicenter phase II trial. J Clin Oncol. 2018;36:446–453. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

