Drug tolerability and reasons for discontinuation of seven biologics in elderly patients with rheumatoid arthritis -The ANSWER cohort study
- PMID: 31067271
- PMCID: PMC6505948
- DOI: 10.1371/journal.pone.0216624
Drug tolerability and reasons for discontinuation of seven biologics in elderly patients with rheumatoid arthritis -The ANSWER cohort study
Abstract
Background: The aim of this study is to evaluate the retention rates and reasons for discontinuation for seven biological disease-modifying antirheumatic drugs (bDMARDs) in a real-world setting of elderly patients (65 years of age or older) with rheumatoid arthritis (RA).
Methods: This multi-center, retrospective study assessed 1,098 treatment courses of 661 patients with bDMARDs from 2009 to 2018 (females, 80.7%; baseline age, 71.7 years; disease duration 10.5 years; rheumatoid factor positivity 81.3%; Disease Activity Score in 28 joints using erythrocyte sedimentation rate, 4.6; concomitant prednisolone dose 2.8 mg/day (45.6%) and methotrexate dose 4.4 mg/week (56.4%); and 60.2% patients were bio-naïve). Treatment courses included abatacept (ABT; n = 272), tocilizumab (TCZ; n = 234), etanercept (ETN; n = 184), golimumab (GLM; n = 159), infliximab (IFX; n = 101), adalimumab (ADA; n = 97), and certolizumab pegol (CZP; n = 51). Drug retention rates and discontinuation reasons were estimated at 36 months using the Kaplan-Meier method and adjusted for potential clinical confounders (age, sex, disease duration, concomitant PSL and MTX, starting date and switched number of bDMARDs) by Cox proportional hazards modeling.
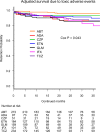
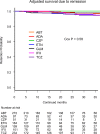
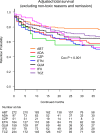
Results: A total of 51.2% of treatment courses were stopped, with 25.1% stopping due to lack of effectiveness, 11.8% due to toxic adverse events, 9.7% due to non-toxic reasons, and 4.6% due to remission. Drug retention rates for each discontinuation reason were as follows; lack of effectiveness [from 55.4% (ETN) to 81.6% (ABT); with significant differences between groups (Cox P<0.001)], toxic adverse events [from 79.3% (IFX) to 95.4% (ABT), Cox P = 0.043], and remission [from 94.2% (TCZ) to 100.0% (CZP), Cox P = 0.58]. Finally, overall retention rates excluding non-toxic reasons and remission for discontinuation ranged from 50.0% (ETN) to 78.1% (ABT) (Cox P<0.001).
Conclusions: ABT showed lowest discontinuation rate by lack of effectiveness and by toxic adverse events, which lead to highest overall retention rates (excluding non-toxic reasons and remission) among seven bDMARDs in adjusted model of elderly RA patients.
Conflict of interest statement
This study was funded by UCB Japan. KE received a research grant and/or speaker fee from Abbvie, Asahi-Kasei, Astellas, Bristol-Myers Squibb, Chugai, Daiichi Sankyo, Eisai, Eli Lilly, Mitsubishi-Tanabe, Ono Pharmaceutical, Pfizer, Taisho-toyama, and UCB Japan. MH and KM are affiliated with a department that is financially supported by four pharmaceutical companies (Mitsubishi-Tanabe, Chugai, Ayumi, and UCB Japan) and the city government (Nagahama City). MH received a research grant and/or speaker fee from Astellas, Mitsubishi-Tanabe, Eisai, and Bristol-Myers Squibb. TH received a research grant from Astellas, and a speaker fee from Astellas, Chugai, Pfizer, Bristol-Myers Squibb, and Takeda. KM received a speaking fee, and/or consulting fee from Eisai. YM received a research grant and/or speaker fee from Eli Lilly, Chugai, Pfize, Bristol-Myers Squibb, and Mitsubishi-Tanabe. TT is affiliated with a department that is financially supported by six pharmaceutical companies (Mitsubishi-Tanabe, Chugai, Ayumi, Astellas, Eisai, and Takeda). TT received a research grant from Chugai, Cover Letter and a speaker fee from Astellas, Chugai, Eisai, Mitsubishi-Tanabe, Abbvie, Bristol-Myers Squibb, Ayumi, Daiichi Sankyo, Eisai, Takeda, and Asahi-Kasei. MH received a speaker fee from Astellas, Ono Pharmacetical, Eli Lilly, Mitsubishi-Tanabe, Pfizer, Ayumi, and Takeda. AK received a research grant and/or speaker fee from Mitsubishi-Tanabe, Chugai, Eisai, Asahi-Kasei, Astellas, Abbvie, Bristol-Myers Squibb, Ono Pharmaceutical, Astellas, and Pfizer. HY received a research grant from Chugai, Daiichi Sankyo, and Pfizer. WY, RH, MK, AO, KN, YS, HA, KY, and SJ have no financial conflicts of interest to disclose concerning this manuscript. These companies had no role in the study design, decision to publish, or preparation of the manuscript. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures

References
-
- Favalli EG, Pregnolato F, Biggioggero M, Becciolini A, Penatti AE, Marchesoni A, et al. Twelve-Year Retention Rate of First-Line Tumor Necrosis Factor Inhibitors in Rheumatoid Arthritis: Real-Life Data From a Local Registry. Arthritis Care Res (Hoboken). 2016;68(4):432–9. Epub 2015/11/12. 10.1002/acr.22788 . - DOI - PubMed
-
- Hetland ML, Christensen IJ, Tarp U, Dreyer L, Hansen A, Hansen IT, et al. Direct comparison of treatment responses, remission rates, and drug adherence in patients with rheumatoid arthritis treated with adalimumab, etanercept, or infliximab: results from eight years of surveillance of clinical practice in the nationwide Danish DANBIO registry. Arthritis Rheum. 2010;62(1):22–32. Epub 2009/12/30. 10.1002/art.27227 . - DOI - PubMed
-
- Neovius M, Arkema EV, Olsson H, Eriksson JK, Kristensen LE, Simard JF, et al. Drug survival on TNF inhibitors in patients with rheumatoid arthritis comparison of adalimumab, etanercept and infliximab. Ann Rheum Dis. 2015;74(2):354–60. Epub 2013/11/29. 10.1136/annrheumdis-2013-204128 annrheumdis-2013-204128 [pii]. - DOI - PMC - PubMed
-
- Souto A, Maneiro JR, Gomez-Reino JJ. Rate of discontinuation and drug survival of biologic therapies in rheumatoid arthritis: a systematic review and meta-analysis of drug registries and health care databases. Rheumatology (Oxford). 2016;55(3):523–34. Epub 2015/10/23. 10.1093/rheumatology/kev374 kev374 [pii]. . - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

