Elastin-Dependent Aortic Heart Valve Leaflet Curvature Changes During Cyclic Flexure
- PMID: 31067726
- PMCID: PMC6631801
- DOI: 10.3390/bioengineering6020039
Elastin-Dependent Aortic Heart Valve Leaflet Curvature Changes During Cyclic Flexure
Abstract
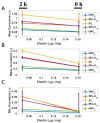
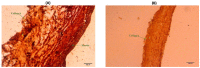
The progression of calcific aortic valve disease (CAVD) is characterized by extracellular matrix (ECM) remodeling, leading to structural abnormalities and improper valve function. The focus of the present study was to relate aortic valve leaflet axial curvature changes as a function of elastin degradation, which has been associated with CAVD. Circumferential rectangular strips (L × W = 10 × 2.5 mm) of normal and elastin-degraded (via enzymatic digestion) porcine AV leaflets were subjected to cyclic flexure (1 Hz). A significant increase in mean curvature (p < 0.05) was found in elastin-degraded leaflet specimens in comparison to un-degraded controls at both the semi-constrained (50% of maximum flexed state during specimen bending and straightening events) and fully-constrained (maximally-flexed) states. This significance did not occur in all three flexed configurations when measurements were performed using either minimum or maximum curvature. Moreover, the mean curvature increase in the elastin-degraded leaflets was most pronounced at the instance of maximum flexure, compared to un-degraded controls. We conclude that the mean axial curvature metric can detect distinct spatial changes in aortic valve ECM arising from the loss in bulk content and/or structure of elastin, particularly when there is a high degree of tissue bending. Therefore, the instance of maximum leaflet flexure during the cardiac cycle could be targeted for mean curvature measurements and serve as a potential biomarker for elastin degradation in early CAVD remodeling.
Keywords: aortic valve; biomarker; calcification; curvature; early detection; elastin degradation; leaflet.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Perrotta I., Camastra C., Filice G., Di Mizio G., Colosimo F., Ricci P., Tripepi S., Amorosi A., Triumbari F., Donato G., et al. New evidence for a critical role of elastin in calcification of native heart valves: Immunohistochemical and ultrastructural study with literature review. Histopathology. 2011;59:504–513. doi: 10.1111/j.1365-2559.2011.03977.x. - DOI - PubMed
-
- Yutzey K.E., Demer L.L., Body S.C., Huggins G.S., Towler D.A., Giachelli C.M., Hofmann-Bowman M.A., Mortlock D.P., Rogers M.B., Sadeghi M.M., et al. Calcific aortic valve disease: A consensus summary from the Alliance of Investigators on Calcific Aortic Valve Disease. Arterioscler. Thromb. Vasc. Biol. 2014;34:2387–2393. doi: 10.1161/ATVBAHA.114.302523. - DOI - PMC - PubMed
-
- Engelmayr G.C., Sales V.L., Mayer J.E., Sacks M.S. Cyclic flexure and laminar flow synergistically accelerate mesenchymal stem cell-mediated engineered tissue formation: Implications for engineered heart valve tissues. Biomaterials. 2006;27:6083–6095. doi: 10.1016/j.biomaterials.2006.07.045. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

