Glioblastoma vs temozolomide: can the red queen race be won?
- PMID: 31068075
- PMCID: PMC6606031
- DOI: 10.1080/15384047.2019.1599662
Glioblastoma vs temozolomide: can the red queen race be won?
Abstract
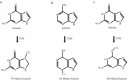
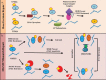
Glioblastoma is the most invasive form of brain tumor. Although temozolomide chemotherapy has been shown to significantly improve survival in patients with GBM, this increase is only trivial. The underlying cause is that many GBMs do not respond to temozolomide, and the rest produces resistance. In the past two decades, many attempts have been made to understand resistance mechanisms and to combine other treatments with temozolomide to maximize patient benefit. Unfortunately, it seems to be a red queen game, and the speed of disease development is as fast as the progress in the field. In order to win this game, a comprehensive approach is needed to decipher the details of the resistance mechanism and to transfer the basic research to the clinic. This article reviews the following: temozolomide discovery, chemistry, and mechanism of action, and mechanisms of resistance, as well as combination therapy with other strategies.
Keywords: BER; DNA repair; MGMT; MMR; Temozolomide; alkylating agents; chemoresistance; glioblastoma; glioma.
Figures

References
-
- O‘Reilly SM, Newlands ES, Glaser MG, Brampton M, Rice-Edwards JM, Illingworth RD, Richards PG, Kennard C, Colquhoun IR, Lewis P.. Temozolomide: a new oral cytotoxic chemotherapeutic agent with promising activity against primary brain tumors. Eur J Cancer. 1993;29A:940–942. - PubMed
-
- Stupp R, Hegi ME, Mason WP, van Den Bent MJ, Taphoorn MJB, Janzer RC, Ludwin SK, Allgeier A, Fisher B, Belanger K, et al. 2009. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomized phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 10:459–466. doi:10.1016/S1470-2045(09)70025-7. - DOI - PubMed
-
- Newlands ES, Stevens MF, Wedge SR, Wheelhouse RT, Brock C. Temozolomide: a review of its discovery, chemical properties, pre-clinical development, and clinical trials. Cancer Treat Rev. 1997;23:35–61. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
