Deacetylation of Miro1 by HDAC6 blocks mitochondrial transport and mediates axon growth inhibition
- PMID: 31068376
- PMCID: PMC6548128
- DOI: 10.1083/jcb.201702187
Deacetylation of Miro1 by HDAC6 blocks mitochondrial transport and mediates axon growth inhibition
Abstract
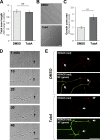
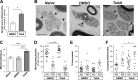
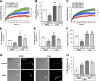
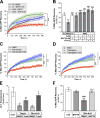
Inhibition of histone deacetylase 6 (HDAC6) was shown to support axon growth on the nonpermissive substrates myelin-associated glycoprotein (MAG) and chondroitin sulfate proteoglycans (CSPGs). Though HDAC6 deacetylates α-tubulin, we find that another HDAC6 substrate contributes to this axon growth failure. HDAC6 is known to impact transport of mitochondria, and we show that mitochondria accumulate in distal axons after HDAC6 inhibition. Miro and Milton proteins link mitochondria to motor proteins for axon transport. Exposing neurons to MAG and CSPGs decreases acetylation of Miro1 on Lysine 105 (K105) and decreases axonal mitochondrial transport. HDAC6 inhibition increases acetylated Miro1 in axons, and acetyl-mimetic Miro1 K105Q prevents CSPG-dependent decreases in mitochondrial transport and axon growth. MAG- and CSPG-dependent deacetylation of Miro1 requires RhoA/ROCK activation and downstream intracellular Ca2+ increase, and Miro1 K105Q prevents the decrease in axonal mitochondria seen with activated RhoA and elevated Ca2+ These data point to HDAC6-dependent deacetylation of Miro1 as a mediator of axon growth inhibition through decreased mitochondrial transport.
© 2019 Kalinski et al.
Figures


Comment in
-
HDAC6 and Miro1: Another interaction causing trouble in neurons.J Cell Biol. 2019 Jun 3;218(6):1769-1770. doi: 10.1083/jcb.201904016. Epub 2019 May 22. J Cell Biol. 2019. PMID: 31118238 Free PMC article.
References
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

