Enveloped viruses distinct from HBV induce dissemination of hepatitis D virus in vivo
- PMID: 31068585
- PMCID: PMC6506506
- DOI: 10.1038/s41467-019-10117-z
Enveloped viruses distinct from HBV induce dissemination of hepatitis D virus in vivo
Abstract
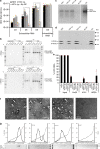
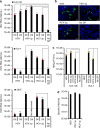
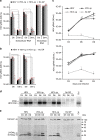
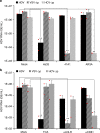
Hepatitis D virus (HDV) doesn't encode envelope proteins for packaging of its ribonucleoprotein (RNP) and typically relies on the surface glycoproteins (GPs) from hepatitis B virus (HBV) for virion assembly, envelopment and cellular transmission. HDV RNA genome can efficiently replicate in different tissues and species, raising the possibility that it evolved, and/or is still able to transmit, independently of HBV. Here we show that alternative, HBV-unrelated viruses can act as helper viruses for HDV. In vitro, envelope GPs from several virus genera, including vesiculovirus, flavivirus and hepacivirus, can package HDV RNPs, allowing efficient egress of HDV particles in the extracellular milieu of co-infected cells and subsequent entry into cells expressing the relevant receptors. Furthermore, HCV can propagate HDV infection in the liver of co-infected humanized mice for several months. Further work is necessary to evaluate whether HDV is currently transmitted by HBV-unrelated viruses in humans.
Conflict of interest statement
The authors declare no competing interests.
Figures


Comment in
-
Master of Disguise: Hepatitis Delta Virus Packaging and Spread Facilitated by Diverse Viral Envelope Proteins.Hepatology. 2020 Jan;71(1):380-382. doi: 10.1002/hep.30922. Epub 2019 Dec 24. Hepatology. 2020. PMID: 31465549 No abstract available.
-
Hepatitis D Virus Infection Revisited.Gastroenterology. 2019 Nov;157(5):1431-1432. doi: 10.1053/j.gastro.2019.09.029. Epub 2019 Oct 3. Gastroenterology. 2019. PMID: 31586567 No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

