Molecular Interplay Between the Sigma-1 Receptor, Steroids, and Ion Channels
- PMID: 31068816
- PMCID: PMC6491805
- DOI: 10.3389/fphar.2019.00419
Molecular Interplay Between the Sigma-1 Receptor, Steroids, and Ion Channels
Abstract
Cell excitability is tightly regulated by the activity of ion channels that allow for the passage of ions across cell membranes. Ion channel activity is controlled by different mechanisms that change their gating properties, expression or abundance in the cell membrane. The latter can be achieved by forming complexes with a diversity of proteins like chaperones such as the Sigma-1 receptor (Sig-1R), which is one with unique features and exhibits a role as a ligand-operated chaperone. This molecule also displays high intracellular mobility according to its activation level since, depletion of internal Ca+2 stores or the presence of specific ligands, produce Sig-1R's mobilization from the endoplasmic reticulum toward the plasma membrane or nuclear envelope. The function of the Sig-1R as a chaperone is regulated by synthetic and endogenous ligands, with some of these compounds being a steroids and acting as key endogenous modifiers of the actions of the Sig-1R. There are cases in the literature that exemplify the close relationship between the actions of steroids on the Sig-1R and the resulting negative or positive effects on ion channel function/abundance. Such interactions have been shown to importantly influence the physiology of mammalian cells leading to changes in their excitability. The present review focuses on describing how the Sig-1R regulates the functional properties and the expression of some sodium, calcium, potassium, and TRP ion channels in the presence of steroids and the physiological consequences of these interplays at the cellular level are also discussed.
Keywords: NMDA – receptor; Sig-1R; TRPV1; ion channels; progesterone; steroids; voltage-gated ion channel.
Figures
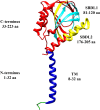
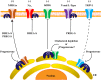


References
-
- Alonso G., Phan V., Guillemain I., Saunier M., Legrand A., Anoal M., et al. (2000). Immunocytochemical localization of the sigma(1) receptor in the adult rat central nervous system. Neuroscience 97 155–170. - PubMed
-
- Aydar E., Palmer C. P., Klyachko V. A., Jackson M. B. (2002). The sigma receptor as a ligand-regulated auxiliary potassium channel subunit. Neuron 34 399–410. - PubMed
-
- Aydar E., Stratton D., Fraser S. P., Djamgoz M. B., Palmer C. (2016). Sigma-1 receptors modulate neonatal Nav1.5 ion channels in breast cancer cell lines. Eur. Biophys. J. 45 671–683. - PubMed
-
- Balasuriya D., D’Sa L., Talker R., Dupuis E., Maurin F., Martin P., et al. (2014). A direct interaction between the sigma-1 receptor and the hERG voltage-gated K+ channel revealed by atomic force microscopy and homogeneous time-resolved fluorescence (HTRF(R)). J. Biol. Chem. 289 32353–32363. 10.1074/jbc.M114.603506 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

