The Mechanical Stimulation of Myotubes Counteracts the Effects of Tumor-Derived Factors Through the Modulation of the Activin/Follistatin Ratio
- PMID: 31068826
- PMCID: PMC6491697
- DOI: 10.3389/fphys.2019.00401
The Mechanical Stimulation of Myotubes Counteracts the Effects of Tumor-Derived Factors Through the Modulation of the Activin/Follistatin Ratio
Abstract
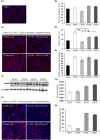
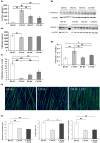
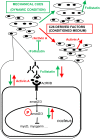
Activin negatively affects muscle fibers and progenitor cells in aging (sarcopenia) and in chronic diseases characterized by severe muscle wasting (cachexia). High circulating activin levels predict poor survival in cancer patients. However, the relative impact of activin in mediating muscle atrophy and hampered homeostasis is still unknown. To directly assess the involvement of activin, and its physiological inhibitor follistatin, in cancer-induced muscle atrophy, we cultured C2C12 myotubes in the absence or in the presence of a mechanical stretching stimulus and in the absence or presence of C26 tumor-derived factors (CM), so as to mimic the mechanical stimulation of exercise and cancer cachexia, respectively. We found that CM induces activin release by myotubes, further exacerbating the negative effects of tumor-derived factors. In addition, mechanical stimulation is sufficient to counteract the adverse tumor-induced effects on muscle cells, in association with an increased follistatin/activin ratio in the cell culture medium, indicating that myotubes actively release follistatin upon stretching. Recombinant follistatin counteracts tumor effects on myotubes exclusively by rescuing fusion index, suggesting that it is only partially responsible for the stretch-mediated rescue. Therefore, besides activin, other tumor-derived factors may play a significant role in mediating muscle atrophy. In addition to increasing follistatin secretion mechanical stimulation induces additional beneficial responses in myotubes. We propose that in animal models of cancer cachexia and in cancer patients purely mechanical stimuli play an important role in mediating the rescue of the muscle homeostasis reported upon exercise.
Keywords: C26 colon carcinoma; FlexCell apparatus; exercise; mechanotransduction; myokines; skeletal muscle atrophy.
Figures
References
-
- Barone R., Macaluso F., Sangiorgi C., Campanella C., Marino Gammazza A., Moresi V., et al. (2016). Skeletal muscle Heat shock protein 60 increases after endurance training and induces peroxisome proliferator-activated receptor gamma coactivator 1 alpha1 expression. Sci. Rep. 6:19781. 10.1038/srep19781 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

