Coagulation Pathways in Neurological Diseases: Multiple Sclerosis
- PMID: 31068896
- PMCID: PMC6491577
- DOI: 10.3389/fneur.2019.00409
Coagulation Pathways in Neurological Diseases: Multiple Sclerosis
Abstract
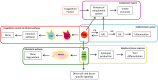
Significant progress has been made in understanding the complex interactions between the coagulation system and inflammation and autoimmunity. Increased blood-brain-barrier (BBB) permeability, a key event in the pathophysiology of multiple sclerosis (MS), leads to the irruption into the central nervous system of blood components that include virtually all coagulation/hemostasis factors. Besides their cytotoxic deposition and role as a possible trigger of the coagulation cascade, hemostasis components cause inflammatory response and immune activation, sustaining neurodegenerative events in MS. Early studies showing the contribution of altered hemostasis in the complex pathophysiology of MS have been strengthened by recent studies using methodologies that permitted deeper investigation. Fibrin(ogen), an abundant protein in plasma, has been identified as a key contributor to neuroinflammation. Perturbed fibrinolysis was found to be a hallmark of progressive MS with abundant cortical fibrin(ogen) deposition. The immune-modulatory function of the intrinsic coagulation pathway still remains to be elucidated in MS. New molecular details in key hemostasis components participating in MS pathophysiology, and particularly involved in inflammatory and immune responses, could favor the development of novel therapeutic targets to ameliorate the evolution of MS. This review article introduces essential information on coagulation factors, inhibitors, and the fibrinolytic pathway, and highlights key aspects of their involvement in the immune system and inflammatory response. It discusses how hemostasis components are (dys)regulated in MS, and summarizes histopathological post-mortem human brain evidence, as well as cerebrospinal fluid, plasma, and serum studies of hemostasis and fibrinolytic pathways in MS. Studies of disease-modifying treatments as potential modifiers of coagulation factor levels, and case reports of autoimmunity affecting hemostasis in MS are also discussed.
Keywords: coagulation; coagulation inhibitors; extrinsic pathway; fibrinolytic pathway; intrinsic pathway; multiple sclerosis.
Figures



References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

