Intra-Familial Phenotypic Heterogeneity and Telomere Abnormality in von Hippel- Lindau Disease: Implications for Personalized Surveillance Plan and Pathogenesis of VHL-Associated Tumors
- PMID: 31068970
- PMCID: PMC6491623
- DOI: 10.3389/fgene.2019.00358
Intra-Familial Phenotypic Heterogeneity and Telomere Abnormality in von Hippel- Lindau Disease: Implications for Personalized Surveillance Plan and Pathogenesis of VHL-Associated Tumors
Abstract
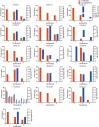
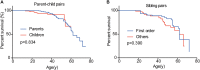
von Hippel-Lindau (VHL) disease is a hereditary cancer syndrome with poor survival. The current recommendations have proposed uniform surveillance strategies for all patients, neglecting the obvious phenotypic varieties. In this study, we aim to confirm the phenotypic heterogeneity in VHL disease and the underlying mechanism. A total of 151 parent-child pairs were enrolled for genetic anticipation analysis, and 77 sibling pairs for birth order effect analysis. Four statistical methods were used to compare the onset age of patients among different generations and different birth orders. The results showed that the average onset age was 18.9 years earlier in children than in their parents, which was statistically significant in all of the four statistical methods. Furthermore, the first-born siblings were affected 8.3 years later than the other ones among the maternal patients. Telomere shortening was confirmed to be associated with genetic anticipation in VHL families, while it failed to explain the birth order effect. Moreover, no significant difference was observed for overall survival between parents and children (p = 0.834) and between first-born patients and the other siblings (p = 0.390). This study provides definitive evidence and possible mechanisms of intra-familial phenotypic heterogeneity in VHL families, which is helpful to the update of surveillance guidelines.
Keywords: birth order effect; genetic anticipation; phenotypic heterogeneity; telomere length; von Hippel-Lindau disease.
Figures




References
-
- Binderup M. L., Bisgaard M. L., Harbud V., Moller H. U., Gimsing S., Friis-Hansen L., et al. (2013). Von Hippel-Lindau disease (vHL). National clinical guideline for diagnosis and surveillance in Denmark. 3rd edition. Dan. Med. J. 60:B4763. - PubMed
LinkOut - more resources
Full Text Sources

