Recent advances in the production of recombinant glycoconjugate vaccines
- PMID: 31069118
- PMCID: PMC6494827
- DOI: 10.1038/s41541-019-0110-z
Recent advances in the production of recombinant glycoconjugate vaccines
Abstract
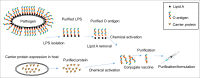
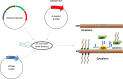
Glycoconjugate vaccines against bacteria are one of the success stories of modern medicine and have led to a significant reduction in the global occurrence of bacterial meningitis and pneumonia. Glycoconjugate vaccines are produced by covalently linking a bacterial polysaccharide (usually capsule, or more recently O-antigen), to a carrier protein. Given the success of glycoconjugate vaccines, it is surprising that to date only vaccines against Haemophilus influenzae type b, Neisseria meningitis and Streptococcus pneumoniae have been fully licenced. This is set to change through the glycoengineering of recombinant vaccines in bacteria, such as Escherichia coli, that act as mini factories for the production of an inexhaustible and renewable supply of pure vaccine product. The recombinant process, termed Protein Glycan Coupling Technology (PGCT) or bioconjugation, offers a low-cost option for the production of pure glycoconjugate vaccines, with the in-built flexibility of adding different glycan/protein combinations for custom made vaccines. Numerous vaccine candidates have now been made using PGCT, which include those improving existing licenced vaccines (e.g., pneumococcal), entirely new vaccines for both Gram-positive and Gram-negative bacteria, and (because of the low production costs) veterinary pathogens. Given the continued threat of antimicrobial resistance and the potential peril of bioterrorist agents, the production of new glycoconjugate vaccines against old and new bacterial foes is particularly timely. In this review, we will outline the component parts of bacterial PGCT, including recent advances, the advantages and limitations of the technology, and future applications and perspectives.
Keywords: Immunology; Pathogenesis.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

