The Role of the Cell Surface Mucin MUC1 as a Barrier to Infection and Regulator of Inflammation
- PMID: 31069176
- PMCID: PMC6491460
- DOI: 10.3389/fcimb.2019.00117
The Role of the Cell Surface Mucin MUC1 as a Barrier to Infection and Regulator of Inflammation
Abstract
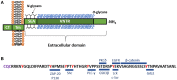
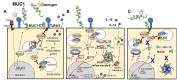
The family of cell surface (cs-) mucins are constitutively expressed at the cell surface by nearly all epithelial cells, beneath the gel-mucin layer. All cs-mucin family members have structural features that enable them to act as a releasable decoy barrier to mucosal pathogens, by providing ligands for pathogen binding and the ability to shed the bound extracellular domain. Due to the towering structure of cs-mucins at the surface, binding of mucosal pathogens can also sterically block binding to underlying cellular receptors. The cytoplasmic tail domain of cs-mucins are capable of initiating signal transduction cascades and due to their conservation across species, may play an important biological role in cellular signaling. MUC1 is one of the most extensively studied of the cs-mucin family. With respect to its physiological function in the mucosal environment, MUC1 has been demonstrated to play a dynamic role in protection of the host from infection by a wide variety of pathogens and to regulate inflammatory responses to infection. This review briefly summarizes the current knowledge and new findings regarding the structural features relating to the function of MUC1, its role as a protective barrier against pathogen invasion and mechanisms by which this cs-mucin regulates inflammation.
Keywords: Campylobacter jejuni; Helicobacter pylori; MUC1; NLRP3-inflammasome; Pseudomonas aeruginosa; Streptococcus pneumoniae; influenza A virus; toll-like receptor.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

