Regulatory switch at the cytoplasmic interface controls TRPV channel gating
- PMID: 31070581
- PMCID: PMC6538378
- DOI: 10.7554/eLife.47746
Regulatory switch at the cytoplasmic interface controls TRPV channel gating
Abstract
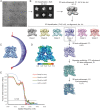
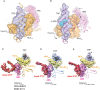
Temperature-sensitive transient receptor potential vanilloid (thermoTRPV) channels are activated by ligands and heat, and are involved in various physiological processes. ThermoTRPV channels possess a large cytoplasmic ring consisting of N-terminal ankyrin repeat domains (ARD) and C-terminal domains (CTD). The cytoplasmic inter-protomer interface is unique and consists of a CTD coiled around a β-sheet which makes contacts with the neighboring ARD. Despite much existing evidence that the cytoplasmic ring is important for thermoTRPV function, the mechanism by which this unique structure is involved in thermoTRPV gating has not been clear. Here, we present cryo-EM and electrophysiological studies which demonstrate that TRPV3 gating involves large rearrangements at the cytoplasmic inter-protomer interface and that this motion triggers coupling between cytoplasmic and transmembrane domains, priming the channel for opening. Furthermore, our studies unveil the role of this interface in the distinct biophysical and physiological properties of individual thermoTRPV subtypes.
Keywords: TRP channel; cryo-electronmicroscopy; electrophysiology; human; ion channel; ligand dependent gating; molecular biophysics; sensitization; structural biology.
Conflict of interest statement
LZ, WB, AH, MB, SL No competing interests declared
Figures

















References
-
- Adams PD, Afonine PV, Bunkóczi G, Chen VB, Davis IW, Echols N, Headd JJ, Hung LW, Kapral GJ, Grosse-Kunstleve RW, McCoy AJ, Moriarty NW, Oeffner R, Read RJ, Richardson DC, Richardson JS, Terwilliger TC, Zwart PH. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallographica Section D Biological Crystallography. 2010;66:213–221. doi: 10.1107/S0907444909052925. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources

