Single nucleotide polymorphisms in the human ATP7B gene modify the properties of the ATP7B protein
- PMID: 31070637
- PMCID: PMC6878656
- DOI: 10.1039/c9mt00057g
Single nucleotide polymorphisms in the human ATP7B gene modify the properties of the ATP7B protein
Erratum in
-
Correction: Single nucleotide polymorphisms in the human ATP7B gene modify the properties of the ATP7B protein.Metallomics. 2019 Aug 1;11(8):1441. doi: 10.1039/c9mt90028d. Epub 2019 Aug 2. Metallomics. 2019. PMID: 31372605
Abstract
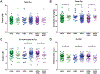
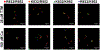
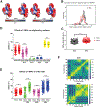
Single nucleotide polymorphisms (SNPs) are the largest source of sequence variation in the human genome. However, their functional significance is not well understood. We show that SNPs in the Wilson disease gene, ATP7B, that produce amino-acid substitutions K832R and R952K, modulate ATP7B properties in vitro and influence serum copper (Cu) status in vivo. The presence of R832 is associated with a lower ATP7B abundance and a diminished trafficking in response to elevated Cu. The K832R substitution alters surface exposure of amino acid residues in the actuator domain and increases its conformational flexibility. All SNP-related ATP7B variants (R832/R952, R832/K952, K832/K952, and K832/R952) have Cu-transport activity. However, the activity of ATP7B-K832/K952 is lower compared to other variants. In humans, the presence of K952 is associated with a higher fraction of exchangeable Cu in serum. Thus, SNPs may modulate the properties of ATP7B and the organism Cu status.
Conflict of interest statement
CONFLICTS OF INTEREST
Dr. R. Squitti is a consultant Canox4drug SPA (Italy) and Igea Research Inc. (Miami, FL). Other authors have no conflicts of interest to declare.
Figures
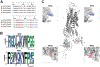

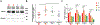
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

