Multimodal Imaging in Best Vitelliform Macular Dystrophy
- PMID: 31070670
- PMCID: PMC6735800
- DOI: 10.1167/iovs.19-26571
Multimodal Imaging in Best Vitelliform Macular Dystrophy
Abstract
Purpose: In patients diagnosed with Best vitelliform macular dystrophy (BVMD), quantitative fundus autofluorescence (qAF), near-infrared fundus autofluorescence (NIR-AF), and spectral-domain optical coherence tomography (SD-OCT) were used to elucidate pathogenic mechanisms.
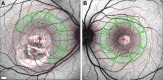
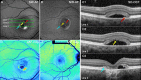
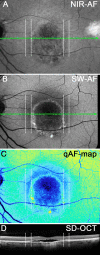
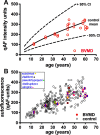
Methods: Fourteen patients heterozygous for BEST1 mutations were recruited. qAF was analyzed using short-wavelength fundus autofluorescence (SW-AF) images. Mean gray levels (GL) were determined in nonlesion areas (7 to 9° eccentricity) and adjusted by GL measured in an internal fluorescent reference. NIR-AF images (787 nm; sensitivity of 96) were captured and saved in non-normalized mode. Horizontal SD-OCT images also were acquired and BVMD was staged according to the OCT findings.
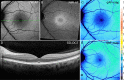
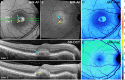
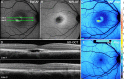
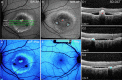
Results: In the pre-vitelliform stage, NIR-AF imaging revealed an area of reduced fluorescence, whereas in the vitelliruptive stage, puncta of elevated NIR-AF signal were present. In both SW-AF and NIR-AF images, the vitelliform lesion in the atrophic stage was marked by reduced signal. At all stages of BVMD, nonlesion qAF was within the 95% confidence intervals for healthy eyes. Similarly, the NIR-AF intensity measurements outside the vitelliform lesion were comparable to the healthy control eye. SD-OCT scans revealed a fluid-filled detachment between the ellipsoid zone and the hyperreflectivity band attributable to RPE/Bruch's membrane.
Conclusions: NIR-AF imaging can identify the pre-vitelliform stage of BVMD. Mutations in BEST1 are not associated with increased levels of SW-AF outside the vitelliform lesion. Elevated SW-AF within the fluid-filled lesion likely reflects the inability of RPE to phagocytose outer segments due to separation of RPE from photoreceptor cells, together with progressive photoreceptor cell impairment.
Figures


References
-
- Marmorstein AD, Marmorstein LY, Rayborn M, Wang XD, Hollyfield JG, Petrukhin K. Bestrophin, the product of the Best vitelliform macular dystrophy gene (VMD2), localizes to the basolateral plasma membrane of the retinal pigment epithelium. Proc Natl Acad Sci U S A. 2000;97:12758–12763. - PMC - PubMed
-
- Boon CJ, Klevering BJ, Leroy BP, Hoyng CB, Keunen JE, den Hollander AI. The spectrum of ocular phenotypes caused by mutations in the BEST1 gene. Prog Retin Eye Res. 2009;28:187–205. - PubMed
-
- Marquardt A, Stohr H, Passmore LA, Kramer F, Rivera A, Weber BH. Mutations in a novel gene, VMD2, encoding a protein of unknown properties cause juvenile-onset vitelliform macular dystrophy (Best's disease) Hum Mol Genet. 1998;7:1517–1525. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

