Life-Course Contribution of Prenatal Stress in Regulating the Neural Modulation Network Underlying the Prepulse Inhibition of the Acoustic Startle Reflex in Male Alzheimer's Disease Mice
- PMID: 31070710
- PMCID: PMC7029700
- DOI: 10.1093/cercor/bhz089
Life-Course Contribution of Prenatal Stress in Regulating the Neural Modulation Network Underlying the Prepulse Inhibition of the Acoustic Startle Reflex in Male Alzheimer's Disease Mice
Abstract
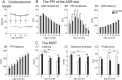
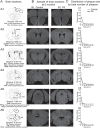
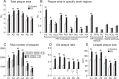
The prepulse inhibition (PPI) of the acoustic startle reflex (ASR), as an index of sensorimotor gating, is one of the most extensively used paradigms in the field of neuropsychiatric disorders. Few studies have examined how prenatal stress (PS) regulates the sensorimotor gating during the lifespan and how PS modifies the development of amyloid-beta (Aβ) pathology in brain areas underlying the PPI formation. We followed alternations in corticosterone levels, learning and memory, and the PPI of the ASR measures in APPNL-G-F/NL-G-F offspring of dams exposed to gestational noise stress. In-depth quantifications of the Aβ plaque accumulation were also performed at 6 months. The results indicated an age-dependent deterioration of sensorimotor gating, long-lasting PS-induced abnormalities in PPI magnitudes, as well as deficits in spatial memory. The PS also resulted in a higher Aβ aggregation predominantly in brain areas associated with the PPI modulation network. The findings suggest the contribution of a PS-induced hypothalamic-pituitary-adrenal (HPA) axis hyperactivity in regulating the PPI modulation substrates leading to the abnormal development of the neural protection system in response to disruptive stimuli. The long-lasting HPA axis dysregulation appears to be the major underlying mechanism in precipitating the Aβ deposition, especially in brain areas contributed to the PPI modulation network.
Keywords: Acoustic startle reflex; Alzheimer's disease; HPA axis; amyloid-beta plaque; corticosterone; noise; prenatal stress; prepulse inhibition; sensorimotor gating.
© The Author(s) 2019. Published by Oxford University Press. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures

References
-
- Abel K, Waikar M, Pedro B, Hemsley D, Geyer M. 1998. Repeated testing of prepulse inhibition and habituation of the startle reflex: a study in healthy human controls. J Psychopharmacol. 12:330–337. - PubMed
-
- Antonelli MC, Pallares ME, Ceccatelli S, Spulber S. 2017. Long-term consequences of prenatal stress and neurotoxicants exposure on neurodevelopment. Prog Neurobiol. 155:21–35. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

