Hepatitis B infection among pregnant and post-partum women living with HIV and on antiretroviral therapy in Kinshasa, DR Congo: A cross-sectional study
- PMID: 31071145
- PMCID: PMC6508921
- DOI: 10.1371/journal.pone.0216293
Hepatitis B infection among pregnant and post-partum women living with HIV and on antiretroviral therapy in Kinshasa, DR Congo: A cross-sectional study
Abstract
Background: Hepatitis B virus (HBV) co-infection in HIV-infected individuals increases the risk of hepatic complications and mortality. Further, the risk of perinatal HBV transmission increases among HBV/HIV co-infected pregnant women. Although HBV is endemic in the Democratic Republic of Congo, there is little data on HBV/HIV co-infection. We aimed to assess the burden and risk factors of HBV surface antigen (HBsAg) positivity among HIV-infected pregnant and post-partum women.
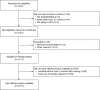
Methods: This cross-sectional study was conducted as part of an ongoing trial to assess the effect of data-driven continuous quality improvement interventions (CQI) for optimal prevention of mother-to-child transmission (PMTCT) of HIV (CQI-PMTCT study, NCT03048669). In each of the 35 health zones of Kinshasa province, all HIV-infected pregnant or breastfeeding women (≤1 year post-delivery) presenting for care in one of the three busiest maternal and child health clinics of the health zone were tested for HBsAg using Alere Determine, Japan. We used logistic regression with general estimating equation accounting for within-clinic clustering to assess risk factors of HBsAg positivity.
Results: Between November 2016 and June 2018, a total of 1377 women, all on antiretroviral therapy, were tested for HBsAg. Overall, 4.7% [95% binomial confidence interval (CI): 3.7%-5.7%] tested positive for HBsAg. HBsAg prevalence was 3.3% (95% CI: 2.1%-4.8%) for women tested during pregnancy, 4.5% (2.5%-7.4%) for those tested at delivery, and 8.5% (5.6%-12.2%) for those tested post-partum (Ptrend = 0.001). In multivariate models including socio-economic status (SES), type of care facility, duration of antiretroviral therapy, HIV viral load, and self-reported intimate partner violence (IPV), lowest tertile of SES, ≤ 6 months of ART, and IPV were all consistently and positively associated with higher prevalence of HBsAg across pregnancy, delivery, and postpartum period while been tested in a health centre or having a viral load ≥ 1000 copies/mL were consistently associated with lower prevalence. However, only the association with IPV (OR = 2.74, 95% CI: 1.10-6.84) and viral load between 40-1000 copies/ml (OR = 4.28, 95% CI: 1.22-15.01) achieved statistical significance among pregnant women.
Conclusion: This study revealed an overall high prevalence of HBsAg among HIV-infected pregnant and post-partum women in Kinshasa with the latter showing the highest HBsAg prevalence. Among pregnant women, intimate partner violence was independently and statistically associated with HBsAg positivity, requiring further investigation.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- Organization WH. Global hepatitis report 2017: World Health Organization; 2017 2017.
-
- Organization WH. World Health OrganizationHepatitis B factsheet. World Health Organization: Geneva: 2017.
-
- Shindano TA, Kabinda JM, Mitashi P, Horsmans Y. Hepatitis B virus infection in the Democratic Republic of Congo: a systematic review of prevalence studies (2000–2016). J Public Health. 2018:1–9. 10.1007/s10389-018-0894-8 - DOI
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical