Effects of Serotonin and Slow-Release 5-Hydroxytryptophan on Gastrointestinal Motility in a Mouse Model of Depression
- PMID: 31071306
- PMCID: PMC6650329
- DOI: 10.1053/j.gastro.2019.04.022
Effects of Serotonin and Slow-Release 5-Hydroxytryptophan on Gastrointestinal Motility in a Mouse Model of Depression
Abstract
Background & aims: Mood disorders and constipation are often comorbid, yet their shared etiologies have rarely been explored. The neurotransmitter serotonin (5-HT) regulates central nervous system and enteric nervous system (ENS) development and long-term functions, including gastrointestinal (GI) motility and mood. Therefore, defects in neuron production of 5-HT might result in brain and intestinal dysfunction. Tryptophan hydroxylase 2 (TPH2) is the rate-limiting enzyme in 5-HT biosynthesis. A variant of TPH2 that encodes the R441H substitution (TPH2-R441H) was identified in individuals with severe depression. We studied mice with an analogous mutation (TPH2-R439H), which results in a 60%-80% decrease in levels of 5-HT in the central nervous system and behaviors associated with depression in humans. Feeding chow that contains 5-HTP slow release (5-HTP SR) to TPH2-R439H mice restores levels of 5-HT in the central nervous system and reduces depressive-like behaviors.
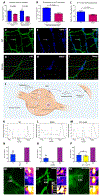
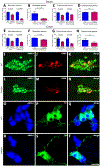
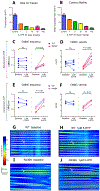
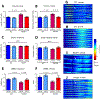
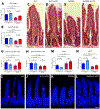
Methods: We compared the effects of feeding chow, with or without 5-HTP SR, to mice with the TPH2-R439H mutation and without this mutation (control mice). Myenteric and submucosal plexuses were isolated from all 4 groups of mice, and immunocytochemistry was used to quantify total enteric neurons, serotonergic neurons, and 5-HT-dependent subsets of neurons. We performed calcium imaging experiments to evaluate responses of enteric neurons to tryptamine-evoked release of endogenous 5-HT. In live mice, we measured total GI transit, gastric emptying, small intestinal transit, and propulsive colorectal motility. To measure colonic migrating motor complexes (CMMCs), we isolated colons and constructed spatiotemporal maps along the proximodistal length to quantify the frequency, velocity, and length of CMMCs. We measured villus height, crypt perimeter, and relative densities of enterochromaffin and enteroendocrine cells in small intestinal tissue.
Results: Levels of 5-HT were significantly lower in enteric neurons from TPH2-R439H mice than from control mice. TPH2-R439H mice had abnormalities in ENS development and ENS-mediated GI functions, including reduced motility and intestinal epithelial growth. Total GI transit and propulsive colorectal motility were slower in TPH2-R439H mice than controls, and CMMCs were slower and less frequent. Villus height and crypt perimeter were significantly decreased in colon tissues from TPH2-R439H mice compared with controls. Administration of 5-HTP SR to adult TPH2-R439H mice restored 5-HT to enteric neurons and reversed these abnormalities. Adult TPH2-R439H mice given oral 5-HTP SR had normalized numbers of enteric neurons, total GI transit, and colonic motility. Intestinal tissue from these mice had normal measures of CMMCs and enteric epithelial growth CONCLUSIONS: In studies of TPH2-R439H mice, we found evidence for reduced release of 5-HT from enteric neurons that results in defects in ENS development and GI motility. Our findings indicate that neuron production of 5-HT links constipation with mood dysfunction. Administration of 5-HTP SR to mice restored 5-HT to the ENS and normalized GI motility and growth of the enteric epithelium. 5-HTP SR might be used to treat patients with intestinal dysfunction associated with low levels of 5-HT.
Keywords: CNS; GI Dysfunction; Gut–Brain Disease; Mood Disorders.
Copyright © 2019 AGA Institute. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures


References
-
- Brody DJ, Pratt LA, Hughes JP. Prevalence of Depression Among Adults Aged 20 and Over: United States, 2013-2016. NCHS Data Brief 2018:1–8. - PubMed
-
- Fond G, Loundou A, Hamdani N, et al. Anxiety and depression comorbidities in irritable bowel syndrome (IBS): a systematic review and meta-analysis. Eur Arch Psychiatry Clin Neurosci 2014;264:651–60. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

