A marijuana-drug interaction primer: Precipitants, pharmacology, and pharmacokinetics
- PMID: 31071346
- PMCID: PMC6708768
- DOI: 10.1016/j.pharmthera.2019.05.001
A marijuana-drug interaction primer: Precipitants, pharmacology, and pharmacokinetics
Abstract
In the United States, the evolving landscape of state-legal marijuana use for recreational and/or medical purposes has given rise to flourishing markets for marijuana and derivative products. The popularity of these products highlights the relative absence of safety, pharmacokinetic, and drug interaction data for marijuana and its constituents, most notably the cannabinoids. This review articulates current issues surrounding marijuana terminology, taxonomy, and dosing; summarizes cannabinoid pharmacology and pharmacokinetics; and assesses the drug interaction risks associated with co-consuming marijuana with conventional medications. Existing pharmacokinetic data are currently insufficient to fully characterize potential drug interactions precipitated by marijuana constituents. As such, increasing awareness among researchers, clinicians, and federal agencies regarding the need to conduct well-designed in vitro and clinical studies is imperative. Mechanisms that help researchers navigate the legal and regulatory barriers to conducting these studies would promote rigorous evaluation of potential marijuana-drug interactions and inform health care providers and consumers about the possible risks of co-consuming marijuana products with conventional medications.
Keywords: Cannabinoid; Drug interaction; Marijuana; Natural product; Pharmacokinetics.
Copyright © 2019 Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

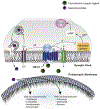
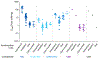

References
-
- Agurell S, Halldin M, Lindgren JE, Ohlsson A, Widman M, Gillespie H, et al. (1986). Pharmacokinetics and metabolism of delta 1-tetrahydrocannabinol and other cannabinoids with emphasis on man. Pharmacol Rev, 38, 21–43. - PubMed
-
- Al Saabi A, Allorge D, Sauvage FL, Tournel G, Gaulier JM, Marquet P, et al. (2013). Involvement of UDP-glucuronosyltransferases UGT1A9 and UGT2B7 in ethanol glucuronidation, and interactions with common drugs of abuse. Drug Metab Dispos, 41, 568–574. - PubMed
-
- Arnold JC, Hone P, Holland ML, & Allen JD (2012). CB2 and TRPV1 receptors mediate cannabinoid actions on MDR1 expression in multidrug resistant cells. Pharmacol Rep, 64, 751–757. - PubMed
-
- Awasthi R, An G, Donovan MD, & Boles Ponto LL (2018). Relating Observed Psychoactive Effects to the Plasma Concentrations of Delta-9-Tetrahydrocannabinol and Its Active Metabolite: An Effect-Compartment Modeling Approach. J Pharm Sci, 107, 745–755. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

