A novel anti-platelet aggregation target of chinensinaphthol methyl ether and neojusticin B obtained from Rostellularia procumbens (L.) Nees
- PMID: 31072143
- PMCID: PMC6522982
- DOI: 10.1080/14756366.2019.1609468
A novel anti-platelet aggregation target of chinensinaphthol methyl ether and neojusticin B obtained from Rostellularia procumbens (L.) Nees
Abstract
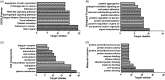
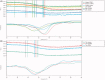
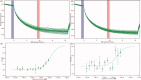
This study explored the possible bioactive ingredients and target protein of Rostellularia procumbens (L.) Nees. The results of optical turbidimetry revealed that the ethyl acetate extraction obtained from R. procumbens (L.) Nees could inhibit platelet aggregation. Gene chip was used to investigate differentially expressed genes. According to the results of the gene chip, the targets of compounds isolated from the ethyl acetate extraction were predicted by network pharmacology. Computational studies revealed that chinensinaphthol methyl ether and neojusticin B may target the integrin αIIbβ3 protein. The results of Prometheus NT.48 and microscale thermophoresis suggested that the molecular interactions between the two compounds with purified integrin αIIbβ3 protein in the optimal test conditions were coherent with the docking results. To our best knowledge, this is the first report to state that chinensinaphthol methyl ether and neojusticin B target the integrin αIIbβ3 protein.
Keywords: Chinensinaphthol methyl ether; Prometheus NT.48; gene chip; integrin α; microscale thermophoresis; neojusticin B; network pharmacology; platelet aggregation.
Figures



References
-
- Andrews RK, Gardiner EE. Basic mechanisms of platelet receptor shedding. Platelets 2017;28:319–24. - PubMed
-
- Chatterjee M, Gawaz M. Clinical significance of receptor shedding-platelet GPVI as an emerging diagnostic and therapeutic tool. Platelets 2017;28:362–71. - PubMed
-
- Bender M, Stegner D, Nieswandt B. Model systems for platelet receptor shedding. Platelets 2017;28:325–32. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
