Late Gadolinium Enhancement Cardiac Magnetic Resonance Tissue Characterization for Cancer-Associated Cardiac Masses: Metabolic and Prognostic Manifestations in Relation to Whole-Body Positron Emission Tomography
- PMID: 31072171
- PMCID: PMC6585339
- DOI: 10.1161/JAHA.118.011709
Late Gadolinium Enhancement Cardiac Magnetic Resonance Tissue Characterization for Cancer-Associated Cardiac Masses: Metabolic and Prognostic Manifestations in Relation to Whole-Body Positron Emission Tomography
Abstract
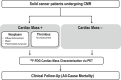
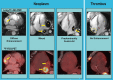
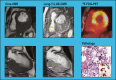
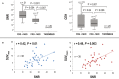
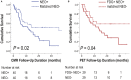
Background Cardiac magnetic resonance ( CMR) differentiates neoplasm from thrombus via contrast enhancement; positron emission tomography ( PET) assesses metabolism. The relationship between CMR contrast enhancement and metabolism on PET is unknown. Methods and Results The population included 121 cancer patients undergoing CMR and 18F-fluorodeoxyglucose (18F- FDG) - PET , including 66 with cardiac masses and cancer-matched controls. Cardiac mass etiology (neoplasm, thrombus) on CMR was defined by late gadolinium enhancement; PET was read blinded to CMR for diagnostic performance, then colocalized to measure FDG avidity. Of CMR -evidenced thrombi (all nonenhancing), none were detected by PET . For neoplasm, PET yielded reasonable sensitivity (70-83%) and specificity (75-88%). Lesions undetected by PET were more likely to be highly mobile ( P=0.001) despite similar size ( P=0.33). Among nonmobile neoplasms, PET sensitivity varied in relation to extent of CMR -evidenced avascularity; detection of diffusely enhancing or mixed lesions was higher versus predominantly avascular neoplasms (87% versus 63%). Colocalized analyses demonstrated 2- to 4-fold higher FDG uptake in neoplasm versus thrombus ( P<0.001); FDG uptake decreased stepwise when neoplasms were partitioned based on extent of avascularity on late gadolinium enhancement CMR ( P≤0.001). Among patients with neoplasm, signal-to-noise ratio on late gadolinium enhancement CMR moderately correlated with standardized uptake values on PET ( r=0.42-0.49, P<0.05). Mortality was higher among patients with CMR -evidenced neoplasm versus controls (hazard ratio: 1.99 [95% CI, 1.1-3.6]; P=0.03) despite nonsignificant differences when partitioned via FDG avidity (hazard ratio: 1.56 [95% CI, 0.85-2.74]; P=0.16). Among FDG-positive neoplasms detected concordantly with CMR , mortality risk versus cancer-matched controls was equivalently increased (hazard ratio: 2.12 [95% CI, 1.01-4.44]; P=0.047). Conclusions CMR contrast enhancement provides a criterion for neoplasm that parallels FDG -evidenced metabolic activity and stratifies prognosis. Extent of tissue avascularity on late gadolinium enhancement CMR affects cardiac mass identification by FDG - PET .
Keywords: cardiac magnetic resonance; cardiac neoplasm; cardio‐oncology; positron emission tomography.
Figures


Similar articles
-
Risk stratification of cardiac metastases using late gadolinium enhancement cardiovascular magnetic resonance: prognostic impact of hypo-enhancement evidenced tumor avascularity.J Cardiovasc Magn Reson. 2021 Apr 5;23(1):42. doi: 10.1186/s12968-021-00727-2. J Cardiovasc Magn Reson. 2021. PMID: 33814005 Free PMC article.
-
Prognostic utility of differential tissue characterization of cardiac neoplasm and thrombus via late gadolinium enhancement cardiovascular magnetic resonance among patients with advanced systemic cancer.J Cardiovasc Magn Reson. 2017 Oct 12;19(1):76. doi: 10.1186/s12968-017-0390-2. J Cardiovasc Magn Reson. 2017. PMID: 29025425 Free PMC article.
-
Hybrid Magnetic Resonance Imaging and Positron Emission Tomography With Fluorodeoxyglucose to Diagnose Active Cardiac Sarcoidosis.JACC Cardiovasc Imaging. 2018 Jan;11(1):94-107. doi: 10.1016/j.jcmg.2017.02.021. Epub 2017 Jun 14. JACC Cardiovasc Imaging. 2018. PMID: 28624396 Free PMC article.
-
A systematic review and meta-analysis of 18F-fluorodeoxyglucose positron emission tomography or positron emission tomography/computed tomography for detection of infected prosthetic vascular grafts.J Vasc Surg. 2019 Jul;70(1):307-313. doi: 10.1016/j.jvs.2019.01.051. Epub 2019 Mar 25. J Vasc Surg. 2019. PMID: 30922755
-
The role of cardiac magnetic resonance in diagnosis of cardiac sarcoidosis.Heart Fail Rev. 2021 May;26(3):653-660. doi: 10.1007/s10741-020-10035-z. Epub 2020 Oct 6. Heart Fail Rev. 2021. PMID: 33025413 Review.
Cited by
-
Left ventricular mural thrombus appearing as a photopenic defect on myocardial viability PET imaging.J Nucl Cardiol. 2022 Oct;29(5):2713-2715. doi: 10.1007/s12350-020-02480-y. Epub 2021 Jan 5. J Nucl Cardiol. 2022. PMID: 33403514 No abstract available.
-
[18F]FDG PET imaging in the differentiation of cardiac masses: an updated systematic review and dual Meta-Analysis of diagnostic performance and parameter variability.Eur J Nucl Med Mol Imaging. 2025 Jul;52(9):3379-3394. doi: 10.1007/s00259-025-07289-w. Epub 2025 Apr 29. Eur J Nucl Med Mol Imaging. 2025. PMID: 40298987 Free PMC article.
-
Membranous Ventricular Septal Aneurysm Leading to Embolic Stroke.CASE (Phila). 2022 Mar 4;6(3):142-145. doi: 10.1016/j.case.2022.01.010. eCollection 2022 May. CASE (Phila). 2022. PMID: 35602978 Free PMC article.
-
Risk stratification of cardiac metastases using late gadolinium enhancement cardiovascular magnetic resonance: prognostic impact of hypo-enhancement evidenced tumor avascularity.J Cardiovasc Magn Reson. 2021 Apr 5;23(1):42. doi: 10.1186/s12968-021-00727-2. J Cardiovasc Magn Reson. 2021. PMID: 33814005 Free PMC article.
-
Diagnostic test accuracies of F-18 FDG PET for characterisation of cardiac masses compared to conventional imaging techniques: systematic review and meta-analysis.Br J Radiol. 2022 Jul 1;95(1135):20210263. doi: 10.1259/bjr.20210263. Epub 2022 May 25. Br J Radiol. 2022. PMID: 35612548 Free PMC article.
References
-
- Chan AT, Plodkowski AJ, Pun SC, Lakhman Y, Halpenny DF, Kim J, Goldburg SR, Matasar MJ, Moskowitz CS, Gupta D, Steingart R, Weinsaft JW. Prognostic utility of differential tissue characterization of cardiac neoplasm and thrombus via late gadolinium enhancement cardiovascular magnetic resonance among patients with advanced systemic cancer. J Cardiovasc Magn Reson. 2017;19:76. - PMC - PubMed
-
- Nensa F, Tezgah E, Poeppel TD, Jensen CJ, Schelhorn J, Kohler J, Heusch P, Bruder O, Schlosser T, Nassenstein K. Integrated 18F‐FDG PET/MR imaging in the assessment of cardiac masses: a pilot study. J Nucl Med. 2015;56:255–260. - PubMed
-
- Pun SC, Plodkowski A, Matasar MJ, Lakhman Y, Halpenny DF, Gupta D, Moskowitz C, Kim J, Steingart R, Weinsaft JW. Pattern and prognostic implications of cardiac metastases among patients with advanced systemic cancer assessed with cardiac magnetic resonance imaging. J Am Heart Assoc. 2016;5:e003368 DOI: 10.1161/JAHA.116.003368. - DOI - PMC - PubMed
-
- Rahbar K, Seifarth H, Schafers M, Stegger L, Hoffmeier A, Spieker T, Tiemann K, Maintz D, Scheld HH, Schober O, Weckesser M. Differentiation of malignant and benign cardiac tumors using 18F‐FDG PET/CT. J Nucl Med. 2012;53:856–863. - PubMed
-
- Srichai MB, Junor C, Rodriguez LL, Stillman AE, Grimm RA, Lieber ML, Weaver JA, Smedira NG, White RD. Clinical, imaging, and pathological characteristics of left ventricular thrombus: a comparison of contrast‐enhanced magnetic resonance imaging, transthoracic echocardiography, and transesophageal echocardiography with surgical or pathological validation. Am Heart J. 2006;152:75–84. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

