Combined kyphoplasty and intraoperative radiotherapy (Kypho-IORT) versus external beam radiotherapy (EBRT) for painful vertebral metastases - a randomized phase III study
- PMID: 31072314
- PMCID: PMC6507138
- DOI: 10.1186/s12885-019-5666-5
Combined kyphoplasty and intraoperative radiotherapy (Kypho-IORT) versus external beam radiotherapy (EBRT) for painful vertebral metastases - a randomized phase III study
Abstract
Background: The spine is the most frequent location of bone metastases. Local treatment aims at palliation of pain and, given the increased likelihood of long-term cancer survival, at local control. Kyphoplasty and intraoperative radiotherapy (Kypho-IORT) provided instantaneous pain relief in 70% of patients at the first day after the intervention and resulted in local control rates of > 93% at 1 year in a recently conducted phase I/II trial. To assess its clinical value, we designed a phase III trial which tests Kypho-IORT against the most widespread standard-of-care, external beam radiotherapy (EBRT), in patients with painful vertebral metastases.
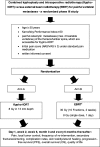
Methods: This phase III study includes patients ≥50 years of age with up to 4 vertebral metastases and a pain score of at least 3/10 points on the visual/numeric analogy scale (VAS). Patients randomized into the experimental arm (A) will undergo Kypho-IORT (Kyphoplasty plus IORT with 8 Gy prescribed to 13 mm depth). Patients randomized into the control arm (B) will receive EBRT with either 30 Gy in 10 fractions or 8 Gy as a single dose. The primary end point is pain reduction defined as at least - 3 points on the VAS compared to baseline at day 1. Assuming that 40% of patients in the Kypho-IORT arm and 5% of patients in the control arm will achieve this reduction and 20% will drop out, a total of 54 patients will have to be included to reach a power of 0.817 with a two-sided alpha of 0.05. Secondary endpoints are evaluation of the percentage of patients with a pain reduction of at least 3 points at 2 and 6 weeks, local tumor control, frequency of re-intervention, secondary fractures/sintering, complication rates, skin toxicity/wound healing, progression-free survival (PFS), overall survival (OS) and quality of life.
Discussion: This trial will generate level 1 evidence on the clinical value of a one-stop procedure which may provide instantaneous pain relief, long-term control and shortened intervals to further adjuvant (systemic) therapies in patients with spinal metastases.
Trial registration: Registered with ClinicalTrials.gov, number: NCT02773966 (Registration date: 05/16/2016).
Keywords: Cement augmentation; External beam radiotherapy; Intraoperative radiotherapy; Kypho-IORT; Kyphoplasty; Vertebral metastases.
Conflict of interest statement
Ethics approval and consent to participate
This study was approved by the local ethics committee (Medical Ethics Commission II of the Faculty of Medicine Mannheim, University of Heidelberg, 2013-593 N-MA) and the Federal Office of Radiation Protection (Z5–22462/2–2013-116). Written informed consent is obtained from all patients participating in this study.
Consent for publication
Not applicable.
Competing interests
FB, UO, ESp, YAM, FS and SC are on the Carl Zeiss Meditec AG speaker’s bureau. GW received travel support from Carl Zeiss Meditec AG. FW is an advisor, consultant and/or speaker for Celgene GmbH, Roche Pharma AG, Eli Lilly and Company, Ipsen Pharma GmbH, receives travel and research grants from Carl Zeiss Meditec AG and Elekta AB, is on the Carl Zeiss Meditec AG speaker’s bureau and holds patents related with Carl Zeiss Meditec AG. FG serves as consultant and speaker for Carl Zeiss Meditec AG (the manufacturer of the device used for IORT in this trial), NOXXON Pharma AG, Merck Serono GmbH, Roche Pharma AG, Siemens Healthcare Diagnostics GmbH and holds patents related with Carl Zeiss Meditec AG.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Kaasa S, Brenne E, Lund JA, Fayers P, Falkmer U, Holmberg M, et al. Prospective randomised multicenter trial on single fraction radiotherapy (8 Gy x 1) versus multiple fractions (3 Gy x 10) in the treatment of painful bone metastases. Radiother Oncol. 2006;79:278–284. doi: 10.1016/j.radonc.2006.05.006. - DOI - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

