Priorities when deciding on participation in early-phase gene therapy trials for Duchenne muscular dystrophy: a best-worst scaling experiment in caregivers and adult patients
- PMID: 31072340
- PMCID: PMC6509771
- DOI: 10.1186/s13023-019-1069-6
Priorities when deciding on participation in early-phase gene therapy trials for Duchenne muscular dystrophy: a best-worst scaling experiment in caregivers and adult patients
Abstract
Purpose: Several gene therapy trials for Duchenne muscular dystrophy initiated in 2018. Trial decision making is complicated by non-curative, time-limited benefits; the progressive, fatal course; and high unmet needs. Here, caregivers and patients prioritize factors influencing decision making regarding participation in early-phase gene therapy trials.
Methods: We conducted a best-worst scaling experiment among U.S. caregivers and adults with Duchenne (N = 274). Participants completed 11 choice sets, choosing features they cared about most and least when deciding whether to participate in a hypothetical gene therapy trial. We analyzed the data using sequential conditional logistic regression.
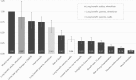
Results: Participants prioritized improved muscle function in trial decision making. Concerns about participation limiting later use of gene transfer and editing were also important, as were improved lung and heart function. Low risk of death fell near the middle. Participants cared least about muscle biopsies and potential for randomization to placebo. Adults with Duchenne and caregivers of non-ambulatory children significantly prioritized improved lung function compared to caregivers of ambulatory children.
Conclusion: Our data demonstrate prioritization of anticipated benefits and opportunity costs relative to potential harms and procedures in gene therapy trial decision making. Such data inform protocol development, education and advocacy efforts, and informed consent.
Keywords: Best-worst scaling; Duchenne muscular dystrophy; Gene therapy; Stated preference.
Conflict of interest statement
Authors’ information
Not applicable.
Ethics approval and consent to participate
The study protocol received IRB review and approval from RTI International’s Committee for the Protection of Human Subjects.
Consent for publication
Not Applicable.
Competing interests
RP– Declares no conflict of interest.
RF– Full-time employee of Parent Project Muscular Dystrophy, which receives funding from Solid Bioscience and Pfizer in support of preference research programs.
CM (1)– Declares no conflict of interest.
BM – Declares no conflict of interest.
KB – Full-time employee of Pfizer Inc. with stock holdings related to employment.
AG – Employee and co-founder of Sold Biosciences, with stock holdings related to employment.
AM – Declares no conflict of interest.
CM (2)– Employee of Solid Biosciences and former employee of Pfizer, with stock holdings related to employment.
CR – Declares no conflict of interest.
VR – Employee of Solid Biosciences with stock holdings related to employment.
LR – Full-time employee of Pfizer Inc. with stock holdings related to employment.
AS – Full-time employee of Pfizer Inc. with stock holdings related to employment.
ECS – Receives salary support from Pfizer Inc. for role as PI on a gene therapy trial.
HP – Declares no conflict of interest.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

