Elasticity of individual protocadherin 15 molecules implicates tip links as the gating springs for hearing
- PMID: 31072932
- PMCID: PMC6561218
- DOI: 10.1073/pnas.1902163116
Elasticity of individual protocadherin 15 molecules implicates tip links as the gating springs for hearing
Abstract
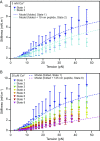
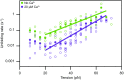
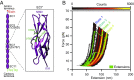
Hair cells, the sensory receptors of the inner ear, respond to mechanical forces originating from sounds and accelerations. An essential feature of each hair cell is an array of filamentous tip links, consisting of the proteins protocadherin 15 (PCDH15) and cadherin 23 (CDH23), whose tension is thought to directly gate the cell's transduction channels. These links are considered far too stiff to represent the gating springs that convert hair bundle displacement into forces capable of opening the channels, and no mechanism has been suggested through which tip-link stiffness could be varied to accommodate hair cells of distinct frequency sensitivity in different receptor organs and animals. Consequently, the gating spring's identity and mechanism of operation remain central questions in sensory neuroscience. Using a high-precision optical trap, we show that an individual monomer of PCDH15 acts as an entropic spring that is much softer than its enthalpic stiffness alone would suggest. This low stiffness implies that the protein is a significant part of the gating spring that controls a hair cell's transduction channels. The tip link's entropic nature then allows for stiffness control through modulation of its tension. We find that a PCDH15 molecule is unstable under tension and exhibits a rich variety of reversible unfolding events that are augmented when the Ca2+ concentration is reduced to physiological levels. Therefore, tip link tension and Ca2+ concentration are likely parameters through which nature tunes a gating spring's mechanical properties.
Keywords: auditory system; entropic stiffness; hair cell; optical trap; vestibular system.
Copyright © 2019 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Zhao Q, et al. (2018) Structure and mechanogating mechanism of the Piezo1 channel. Nature 554:487–492. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

