Disruption of the exocyst induces podocyte loss and dysfunction
- PMID: 31073028
- PMCID: PMC6664173
- DOI: 10.1074/jbc.RA119.008362
Disruption of the exocyst induces podocyte loss and dysfunction
Abstract
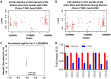
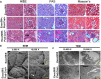
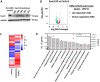
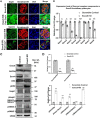
Although the slit diaphragm proteins in podocytes are uniquely organized to maintain glomerular filtration assembly and function, little is known about the underlying mechanisms that participate in trafficking these proteins to the correct location for development and homeostasis. Identifying these mechanisms will likely provide novel targets for therapeutic intervention to preserve podocyte function following glomerular injury. Analysis of structural variation in cases of human nephrotic syndrome identified rare heterozygous deletions of EXOC4 in two patients. This suggested that disruption of the highly-conserved eight-protein exocyst trafficking complex could have a role in podocyte dysfunction. Indeed, mRNA profiling of injured podocytes identified significant exocyst down-regulation. To test the hypothesis that the exocyst is centrally involved in podocyte development/function, we generated homozygous podocyte-specific Exoc5 (a central exocyst component that interacts with Exoc4) knockout mice that showed massive proteinuria and died within 4 weeks of birth. Histological and ultrastructural analysis of these mice showed severe glomerular defects with increased fibrosis, proteinaceous casts, effaced podocytes, and loss of the slit diaphragm. Immunofluorescence analysis revealed that Neph1 and Nephrin, major slit diaphragm constituents, were mislocalized and/or lost. mRNA profiling of Exoc5 knockdown podocytes showed that vesicular trafficking was the most affected cellular event. Mapping of signaling pathways and Western blot analysis revealed significant up-regulation of the mitogen-activated protein kinase and transforming growth factor-β pathways in Exoc5 knockdown podocytes and in the glomeruli of podocyte-specific Exoc5 KO mice. Based on these data, we propose that exocyst-based mechanisms regulate Neph1 and Nephrin signaling and trafficking, and thus podocyte development and function.
Keywords: cilia; ciliopathy; connecting cilium; endocytosis; exocyst; photoreceptor; podocyte; protein trafficking; trafficking.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures




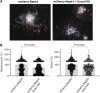
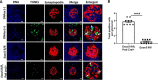
References
-
- Hulkko J., Patrakka J., Lal M., Tryggvason K., Hultenby K., and Wernerson A. (2014) Neph1 is reduced in primary focal segmental glomerulosclerosis, minimal change nephrotic syndrome, and corresponding experimental animal models of adriamycin-induced nephropathy and puromycin aminonucleoside nephrosis. Nephron Extra 4, 146–154 10.1159/000365091 - DOI - PMC - PubMed
-
- Wagner M. C., Rhodes G., Wang E., Pruthi V., Arif E., Saleem M. A., Wean S. E., Garg P., Verma R., Holzman L. B., Gattone V., Molitoris B. A., and Nihalani D. (2008) Ischemic injury to kidney induces glomerular podocyte effacement and dissociation of slit diaphragm proteins Neph1 and ZO-1. J. Biol. Chem. 283, 35579–35589 10.1074/jbc.M805507200 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

