Percutaneous management of enterocutaneous fistulae and abscess-fistula complexes
- PMID: 31073548
- PMCID: PMC6502267
- DOI: 10.1055/s-0038-1660452
Percutaneous management of enterocutaneous fistulae and abscess-fistula complexes
Abstract
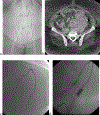
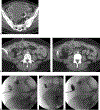
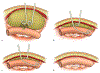
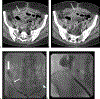
Abscess-fistula complexes and enterocutaneous fistulae are due to postoperative, spontaneous, and inflammatory etiologies. Conservative, percutaneous, endoscopic, and surgical treatment options are available options. Interventional radiologists have an array of different treatment strategies, often starting with percutaneous drainage of associated intra-abdominal abscesses. This review article details different percutaneous management strategies, focusing on percutaneous catheter strategies for abscess-fistula complexes along with tract closures strategies for enterocutaneous fistulae.
Keywords: Abscess-fistula-complex; Crohn disease; enterocutaneous fistula; fistula tract embolization; interventional radiology; percutaneous drainage.
Figures






References
-
- Ballard DH,Hamidian Jahromi A,Li AY,Vea R,Ahuja C, D’Agostino HB. Abscess-fistula complexes: a systematic approach for percutaneous catheter management. J Vasc Interv Radiol 2015;26(09): 1363–1367 - PubMed
-
- Rahman FN, Stavas JM. Interventional radiologic management and treatment of enterocutaneous fistulae. J Vasc Interv Radiol 2015;26(01):7–19, quiz 20 - PubMed
-
- Kerlan RK Jr,Jeffrey RB Jr,Pogany AC,Ring EJ.Abdominalabscess with low-output fistula: successful percutaneous drainage. Radiology 1985;155(01):73–75 - PubMed
-
- Lee SS, Kim AY, Yang S-K, et al. Crohn disease of the small bowel: comparison of CT enterography, MR enterography, and small- bowel follow-through as diagnostic techniques. Radiology 2009; 251(03):751–761 - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
