Safety of Topical Non-steroidal Anti-Inflammatory Drugs in Osteoarthritis: Outcomes of a Systematic Review and Meta-Analysis
- PMID: 31073923
- PMCID: PMC6509095
- DOI: 10.1007/s40266-019-00661-0
Safety of Topical Non-steroidal Anti-Inflammatory Drugs in Osteoarthritis: Outcomes of a Systematic Review and Meta-Analysis
Abstract
Objective: We aimed to assess the safety of topical non-steroidal anti-inflammatory drugs (NSAIDs) in the management of osteoarthritis (OA) in a systematic review and meta-analysis of randomized, placebo-controlled trials.
Methods: A comprehensive literature search was undertaken in the MEDLINE, Cochrane Central Register of Controlled Trials (CENTRAL), and Scopus electronic databases. Randomized, double-blind, placebo-controlled, parallel-group trials that assessed adverse events (AEs) with topical NSAIDs in patients with OA were eligible for inclusion. Authors and/or study sponsors were contacted to obtain the full report of AEs. The primary outcomes were overall severe and serious AEs, as well as the following MedDRA System Organ Class (SOC)-related AEs: gastrointestinal, vascular, cardiac, nervous system, skin and subcutaneous tissue, musculoskeletal and connective tissue.
Results: The search strategy identified 1209 records, from which 25 papers were included in the qualitative synthesis and 19 were included in the meta-analysis, after exclusions. Overall, more total AEs (odds ratio [OR] 1.16, 95% confidence interval [CI] 1.04-1.29; I2 = 0.0%) and more withdrawals due to AEs (OR 1.49, 95% CI 1.15-1.92; I2 = 0.0%) were observed with topical NSAIDs compared with placebo. The same results were achieved with topical diclofenac, largely driven by an increase in skin and subcutaneous tissue disorders (OR 1.73, 95% CI 0.96-3.10), although the difference was not statistically significant compared with placebo. No significant difference in the odds for gastrointestinal disorders was observed between topical NSAIDs and placebo (OR 0.96, 95% CI 0.73-1.27).
Conclusions: Topical NSAIDs may be considered safe in the management of OA, especially with regard to low gastrointestinal toxicity. The use of topical NSAIDs in OA should be considered, taking into account their risk: benefit profile in comparison with other anti-OA treatments.
Conflict of interest statement
Olivier Bruyère reports grants from Biophytis, IBSA, MEDA, Servier, SMB and Theramex, outside of the submitted work. Cyrus Cooper reports personal fees from Alliance for Better Bone Health, Amgen, Eli Lilly, GlaxoSmithKline, Medtronic, Merck, Novartis, Pfizer, Roche, Servier, Takeda and UCB, outside of the submitted work. Jean-Yves Reginster reports grants from IBSA-Genevrier, Mylan, CNIEL and Radius Health (through institution); consulting fees from IBSA-Genevrier, Mylan, CNIEL, Radius Health and Pierre Fabre; fees for participation in review activities from IBSA-Genevrier, MYLAN, CNIEL, Radius Health and Teva; and payment for lectures from AgNovos, CERIN, CNIEL, Dairy Research Council (DRC), Echolight, IBSA-Genevrier, Mylan, Pfizer Consumer Health, Teva and Theramex, outside of the submitted work. Thierry Thomas reports personal fees from Abbvie, Amgen, Arrow, BMS, Chugai, Expanscience, Gilead, HAC-Pharma, LCA, Lilly, Medac, MSD, Pfizer, Thuasne, TEVA and UCB, and grants from Amgen, Bone Therapeutics, Chugai, HAC-Pharma, MSD, Novartis, Pfizer and UCB, outside of the submitted work. Germain Honvo, Nicola Veronese, Victoria Leclercq, Anton Geerinck, Alexia Charles, Charlotte Beaudart and Véronique Rabenda have no disclosures to report.
Figures


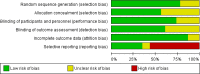
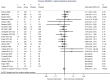


References
-
- Bijlsma JW, Berenbaum F, Lafeber FP. Osteoarthritis: an update with relevance for clinical practice. Lancet. 2011;377(9783):2115–2126. - PubMed
-
- Bruyere O, Cooper C, Pelletier JP, Branco J, Brandi ML, Guillemin F, et al. An algorithm recommendation for the management of knee osteoarthritis in Europe and internationally: a report from a task force of the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO) Semin Arthritis Rheum. 2014;44(3):253–263. - PubMed
-
- McAlindon TE, Bannuru RR, Sullivan MC, Arden NK, Berenbaum F, Bierma-Zeinstra SM, et al. OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthr Cartil. 2014;22(3):363–388. - PubMed
-
- Hochberg MC, Altman RD, April KT, Benkhalti M, Guyatt G, McGowan J, et al. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res. 2012;64(4):465–474. - PubMed
-
- NICE. Osteoarthritis care and management in adults: methods, evidence and recommendations. National Clinical Guideline Centre. Report No. CG177. London: National Institute for Health and Care Excellence; 2014.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

