Vasohibin-2 plays an essential role in metastasis of pancreatic ductal adenocarcinoma
- PMID: 31074083
- PMCID: PMC6609860
- DOI: 10.1111/cas.14041
Vasohibin-2 plays an essential role in metastasis of pancreatic ductal adenocarcinoma
Abstract
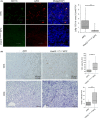
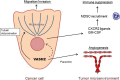
Vasohibin-2 (VASH2) is expressed in various cancers and promotes their progression. We recently reported that pancreatic cancer patients with higher VASH2 expression show poorer prognosis. Herein, we sought to characterize the role of VASH2 in pancreatic cancer. We used LSL-KrasG12D ; LSL-Trp53R172H ; Pdx-1-Cre (KPC) mice, a mouse model of pancreatic ductal adenocarcinoma (PDAC), and cells isolated from them (KPC cells). Knockdown of Vash2 from PDAC cells did not affect their proliferation, but decreased their migration. When Vash2-knockdown PDAC cells were orthotopically inoculated, liver metastasis and peritoneal dissemination were reduced, and the survival period was significantly prolonged. When KPC mice were crossed with Vash2-deficient mice, metastasis was significantly decreased in Vash2-deficient KPC mice. VASH2 was recently identified to have tubulin carboxypeptidase activity. VASH2 knockdown decreased, whereas VASH2 overexpression increased tubulin detyrosination of PDAC cells, and tubulin carboxypeptidase (TCP) inhibitor parthenolide inhibited VASH2-induced cell migration. We next clarified its role in the tumor microenvironment. Tumor angiogenesis was significantly abrogated in vivo when VASH2 was knocked down or deleted. We further examined genes downregulated by Vash2 knockdown in KPC cells, and found chemokines and cytokines that were responsible for the recruitment of myeloid derived suppressor cells (MDSC). Indeed, MDSC were accumulated in PDAC of KPC mice, and they were significantly decreased in Vash2-deficient KPC mice. These findings suggest that VASH2 plays an essential role in the metastasis of PDAC with multiple effects on both cancer cells and the tumor microenvironment, including tubulin detyrosination, tumor angiogenesis and evasion of tumor immunity.
Keywords: MDSC; angiogenesis; pancreatic ductal adenocarcinoma; tubulin detyrosination; vasohibin-2.
© 2019 The Authors. Cancer Science published by John Wiley & Sons Australia, Ltd on behalf of Japanese Cancer Association.
Figures






References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

