RNA sequencing in human HepG2 hepatocytes reveals PPAR-α mediates transcriptome responsiveness of bilirubin
- PMID: 31074682
- PMCID: PMC6620644
- DOI: 10.1152/physiolgenomics.00028.2019
RNA sequencing in human HepG2 hepatocytes reveals PPAR-α mediates transcriptome responsiveness of bilirubin
Abstract
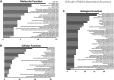
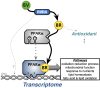
Bilirubin is a potent antioxidant that reduces inflammation and the accumulation of fat. There have been reports of gene responses to bilirubin, which was mostly attributed to its antioxidant function. Using RNA sequencing, we found that biliverdin, which is rapidly reduced to bilirubin, induced transcriptome responses in human HepG2 hepatocytes in a peroxisome proliferator-activated receptor (PPAR)-α-dependent fashion (398 genes with >2-fold change; false discovery rate P < 0.05). For comparison, a much narrower set of genes demonstrated differential expression when PPAR-α was suppressed via lentiviral shRNA knockdown (23 genes). Gene set enrichment analysis revealed the bilirubin-PPAR-α transcriptome mediates pathways for oxidation-reduction processes, mitochondrial function, response to nutrients, fatty acid oxidation, and lipid homeostasis. Together, these findings suggest that transcriptome responses from the generation of bilirubin are mostly PPAR-α dependent, and its antioxidant function regulates a smaller set of genes.
Keywords: PPAR; biliverdin; gene; liver; transcription.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

