Uterine Glands: Developmental Biology and Functional Roles in Pregnancy
- PMID: 31074826
- PMCID: PMC6749889
- DOI: 10.1210/er.2018-00281
Uterine Glands: Developmental Biology and Functional Roles in Pregnancy
Abstract
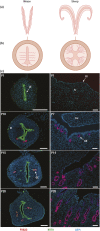
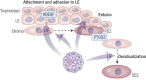
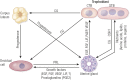
All mammalian uteri contain glands in the endometrium that develop only or primarily after birth. Gland development or adenogenesis in the postnatal uterus is intrinsically regulated by proliferation, cell-cell interactions, growth factors and their inhibitors, as well as transcription factors, including forkhead box A2 (FOXA2) and estrogen receptor α (ESR1). Extrinsic factors regulating adenogenesis originate from other organs, including the ovary, pituitary, and mammary gland. The infertility and recurrent pregnancy loss observed in uterine gland knockout sheep and mouse models support a primary role for secretions and products of the glands in pregnancy success. Recent studies in mice revealed that uterine glandular epithelia govern postimplantation pregnancy establishment through effects on stromal cell decidualization and placental development. In humans, uterine glands and, by inference, their secretions and products are hypothesized to be critical for blastocyst survival and implantation as well as embryo and placental development during the first trimester before the onset of fetal-maternal circulation. A variety of hormones and other factors from the ovary, placenta, and stromal cells impact secretory function of the uterine glands during pregnancy. This review summarizes new information related to the developmental biology of uterine glands and discusses novel perspectives on their functional roles in pregnancy establishment and success.
Copyright © 2019 Endocrine Society.
Figures


References
-
- Gray CA, Bartol FF, Tarleton BJ, Wiley AA, Johnson GA, Bazer FW, Spencer TE. Developmental biology of uterine glands. Biol Reprod. 2001;65(5):1311–1323. - PubMed
-
- Spencer TE, Kelleher AM, Bartol FF. Development and function of uterine glands in domestic animals. Annu Rev Anim Biosci. 2019;7(1):125–147. - PubMed
-
- Bazer FW. Uterine protein secretions: relationship to development of the conceptus. J Anim Sci. 1975;41(5):1376–1382. - PubMed
-
- Roberts RM, Bazer FW. The functions of uterine secretions. J Reprod Fertil. 1988;82(2):875–892. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

