Large-scale molecular phylogeny, morphology, divergence-time estimation, and the fossil record of advanced caenophidian snakes (Squamata: Serpentes)
- PMID: 31075128
- PMCID: PMC6512042
- DOI: 10.1371/journal.pone.0216148
Large-scale molecular phylogeny, morphology, divergence-time estimation, and the fossil record of advanced caenophidian snakes (Squamata: Serpentes)
Erratum in
-
Correction: Large-scale molecular phylogeny, morphology, divergence-time estimation, and the fossil record of advanced caenophidian snakes (Squamata: Serpentes).PLoS One. 2019 May 31;14(5):e0217959. doi: 10.1371/journal.pone.0217959. eCollection 2019. PLoS One. 2019. PMID: 31150527 Free PMC article.
Abstract
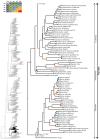
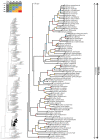
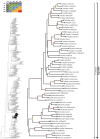
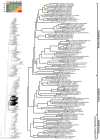
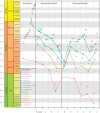
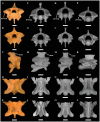
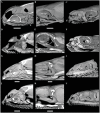
Caenophidian snakes include the file snake genus Acrochordus and advanced colubroidean snakes that radiated mainly during the Neogene. Although caenophidian snakes are a well-supported clade, their inferred affinities, based either on molecular or morphological data, remain poorly known or controversial. Here, we provide an expanded molecular phylogenetic analysis of Caenophidia and use three non-parametric measures of support-Shimodaira-Hasegawa-Like test (SHL), Felsentein (FBP) and transfer (TBE) bootstrap measures-to evaluate the robustness of each clade in the molecular tree. That very different alternative support values are common suggests that results based on only one support value should be viewed with caution. Using a scheme to combine support values, we find 20.9% of the 1265 clades comprising the inferred caenophidian tree are unambiguously supported by both SHL and FBP values, while almost 37% are unsupported or ambiguously supported, revealing the substantial extent of phylogenetic problems within Caenophidia. Combined FBP/TBE support values show similar results, while SHL/TBE result in slightly higher combined values. We consider key morphological attributes of colubroidean cranial, vertebral and hemipenial anatomy and provide additional morphological evidence supporting the clades Colubroides, Colubriformes, and Endoglyptodonta. We review and revise the relevant caenophidian fossil record and provide a time-calibrated tree derived from our molecular data to discuss the main cladogenetic events that resulted in present-day patterns of caenophidian diversification. Our results suggest that all extant families of Colubroidea and Elapoidea composing the present-day endoglyptodont fauna originated rapidly within the early Oligocene-between approximately 33 and 28 Mya-following the major terrestrial faunal turnover known as the "Grande Coupure" and associated with the overall climate shift at the Eocene-Oligocene boundary. Our results further suggest that the caenophidian radiation originated within the Caenozoic, with the divergence between Colubroides and Acrochordidae occurring in the early Eocene, at ~ 56 Mya.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
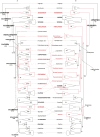
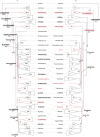
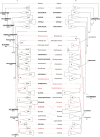


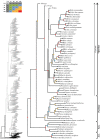



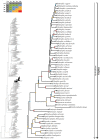
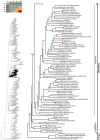


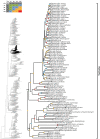
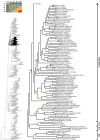






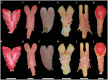

References
-
- Underwood G. A contribution to the classification of snakes. London: British Museum of Natural History; 1967.
-
- Hoffstetter R. Contribution à l’étude des Elapidae actuels et fossils et de l’ostéologie des ophidiens. Arch Mus Hist Nat. Lyon. 1939;15: 1–75.
-
- Haas G. Remarques concernant les relations phylogéniques des diverses familles d’ophidiens fondées sur la différenciation de la musculature mandibulaire. Colloq Int Cent Nat Rech Sci. 1962;104: 215–241.
-
- Hoffstetter R, Gayrard Y. Observations sur l’ostéologie et la classification des Acrochordidae (Serpentes). Bull Mus Natl Hist Nat. 1965;36: 677–696.
-
- Romer AS. Osteology of the reptiles. Chicago: University of Chicago Press; 1956.
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

