ADP heptose, a novel pathogen-associated molecular pattern identified in Helicobacter pylori
- PMID: 31075211
- PMCID: PMC6662969
- DOI: 10.1096/fj.201802555R
ADP heptose, a novel pathogen-associated molecular pattern identified in Helicobacter pylori
Erratum in
-
Corrigendum.FASEB J. 2020 Nov;34(11):15623. doi: 10.1096/fsb2.21084. Epub 2020 Oct 8. FASEB J. 2020. PMID: 33105917 Free PMC article. No abstract available.
Abstract
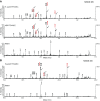
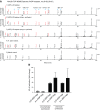
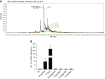
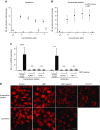
The gastric pathogen Helicobacter pylori activates the NF-κB pathway in human epithelial cells via the recently discovered α-kinase 1 TRAF-interacting protein with forkhead-associated domain (TIFA) axis. We and others showed that this pathway can be triggered by heptose 1,7-bisphosphate (HBP), an LPS intermediate produced in gram-negative bacteria that represents a new pathogen-associated molecular pattern (PAMP). Here, we report that our attempts to identify HBP in lysates of H. pylori revealed surprisingly low amounts, failing to explain NF-κB activation. Instead, we identified ADP-glycero-β-D-manno-heptose (ADP heptose), a derivative of HBP, as the predominant PAMP in lysates of H. pylori and other gram-negative bacteria. ADP heptose exhibits significantly higher activity than HBP, and cells specifically sensed the presence of the β-form, even when the compound was added extracellularly. The data lead us to conclude that ADP heptose not only constitutes the key PAMP responsible for H. pylori-induced NF-κB activation in epithelial cells, but it acts as a general gram-negative bacterial PAMP.-Pfannkuch, L., Hurwitz, R., Traulsen, J., Sigulla, J., Poeschke, M., Matzner, L., Kosma, P., Schmid, M., Meyer, T. F. ADP heptose, a novel pathogen-associated molecular pattern identified in Helicobacter pylori.
Keywords: ALPK1; LPS; NF-κB; PAMP; TIFA.
Conflict of interest statement
The authors thank A. Zamyatina (University of Natural Resources and Life Sciences, Vienna) for providing reference samples of ADP α-heptoses, Meike Sörensen (Max Planck Institute for Infection Biology, Berlin) for creation of plasmids, and Rike Zietlow (Max Planck Institute for Infection Biology, Berlin) for editing the manuscript. The authors declare no conflicts of interest.
Figures

References
-
- Janeway C. A., Jr (1989) Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb. Symp. Quant. Biol. 54, 1–13 - PubMed
-
- Medzhitov R. (2007) Recognition of microorganisms and activation of the immune response. Nature 449, 819–826 - PubMed
-
- Baccala R., Gonzalez-Quintial R., Lawson B. R., Stern M. E., Kono D. H., Beutler B., Theofilopoulos A. N. (2009) Sensors of the innate immune system: their mode of action. Nat. Rev. Rheumatol. 5, 448–456 - PubMed
-
- DiDonato J. A., Mercurio F., Karin M. (2012) NF-κB and the link between inflammation and cancer. Immunol. Rev. 246, 379–400 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

