Enhanced Photocatalytic Degradation of Organic Dyes via Defect-Rich TiO2 Prepared by Dielectric Barrier Discharge Plasma
- PMID: 31075936
- PMCID: PMC6567862
- DOI: 10.3390/nano9050720
Enhanced Photocatalytic Degradation of Organic Dyes via Defect-Rich TiO2 Prepared by Dielectric Barrier Discharge Plasma
Abstract
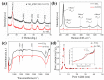
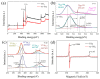
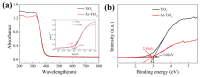
The dye wastewater produced in the printing and dyeing industry causes serious harm to the natural environment. TiO2 usually shows photocatalytic degradation of dye under the irradiation ultravilet light rather than visible light. In this work, a large number of oxygen vacancies and Ti3+ defects were generated on the surface of the TiO2 nanoparticles via Ar plasma. Compared with pristine TiO2 nanoparticles, the as-obtained Ar plasma-treated TiO2 (Ar-TiO2) nanoparticles make the energy band gap reduce from 3.21 eV to 3.17 eV and exhibit enhanced photocatalytic degradation of organic dyes. The Ar-TiO2 obtained exhibited excellent degradation properties of methyl orange (MO); the degradation rate under sunlight irradiation was 99.6% in 30 min, and the photocatalytic performance was about twice that of the original TiO2 nanoparticles (49%). The degradation rate under visible light (λ > 400 nm) irradiation was 89% in 150 min, and the photocatalytic performance of the Ar-TiO2 was approaching ~4 times higher than that of the original TiO2 nanoparticles (23%). Ar-TiO2 also showed good degradation performance in degrading rhodamine B (Rho B) and methylene blue (MB). We believe that this plasma strategy provides a new method for improving the photocatalytic activity of other metal oxides.
Keywords: defects; organic dye; photocatalytic degradation; plasma; titanium dioxide.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Wang Y., Zhu M., Li Y., Zhang M., Xue X., Shi Y., Dai B., Guo X., Feng Y. Heteroatom-doped porous carbon from methyl orange dye wastewater for oxygen reduction. Green Energy Environ. 2017;3:172–178. doi: 10.1016/j.gee.2017.06.005. - DOI
-
- Song C., Zhao J., Li H., Liu L., Xuan L., Xing H., Liu H., Yu Y. One-pot synthesis and combined use of modified cotton adsorbent and flocculant for purifying dyeing wastewater. ACS Sustain. Chem. Eng. 2018;6:6876–6888. doi: 10.1021/acssuschemeng.8b00713. - DOI
-
- Vakili M., Rafatullah M., Salamatinia B., Abdullah A.Z., Ibrahim M.H., Tan K.B., Gholami Z., Amouzgar P. Application of chitosan and its derivatives as adsorbents for dye removal from water and wastewater: A review. Carbohydr. Polym. 2014;113:115–130. doi: 10.1016/j.carbpol.2014.07.007. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

