Pharmaceutical Applications of Molecular Tweezers, Clefts and Clips
- PMID: 31075983
- PMCID: PMC6539068
- DOI: 10.3390/molecules24091803
Pharmaceutical Applications of Molecular Tweezers, Clefts and Clips
Abstract
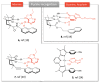
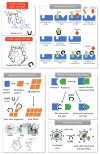
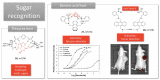
Synthetic acyclic receptors, composed of two arms connected with a spacer enabling molecular recognition, have been intensively explored in host-guest chemistry in the past decades. They fall into the categories of molecular tweezers, clefts and clips, depending on the geometry allowing the recognition of various guests. The advances in synthesis and mechanistic studies have pushed them forward to pharmaceutical applications, such as neurodegenerative disorders, infectious diseases, cancer, cardiovascular disease, diabetes, etc. In this review, we provide a summary of the synthetic molecular tweezers, clefts and clips that have been reported for pharmaceutical applications. Their structures, mechanism of action as well as in vitro and in vivo results are described. Such receptors were found to selectively bind biological guests, namely, nucleic acids, sugars, amino acids and proteins enabling their use as biosensors or therapeutics. Particularly interesting are dynamic molecular tweezers which are capable of controlled motion in response to an external stimulus. They proved their utility as imaging agents or in the design of controlled release systems. Despite some issues, such as stability, cytotoxicity or biocompatibility that still need to be addressed, it is obvious that molecular tweezers, clefts and clips are promising candidates for several incurable diseases as therapeutic agents, diagnostic or delivery tools.
Keywords: biosensing; clefts; clips; controlled release; drug delivery; imaging; molecular switches; molecular tweezers; responsive systems.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

