Microbiota Metabolite Short-Chain Fatty Acids Facilitate Mucosal Adjuvant Activity of Cholera Toxin through GPR43
- PMID: 31076530
- PMCID: PMC6581581
- DOI: 10.4049/jimmunol.1801068
Microbiota Metabolite Short-Chain Fatty Acids Facilitate Mucosal Adjuvant Activity of Cholera Toxin through GPR43
Abstract
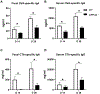
The gut microbiota has been shown critical for mucosal adjuvant activity of cholera toxin (CT), a potent mucosal adjuvant. However, the mechanisms involved remain largely unknown. In this study, we report that depletion of gut bacteria significantly decreased mucosal and systemic Ab responses in mice orally immunized with OVA and CT. Feeding mice short-chain fatty acids (SCFAs) promoted Ab responses elicited by CT, and, more importantly, rescued Ab responses in antibiotic-treated mice. In addition, mice deficient in GPR43, a receptor for SCFAs, showed impaired adjuvant activity of CT. Administering CT did not promote SCFA production in the intestines; thus, SCFAs facilitated but did not directly mediate the adjuvant activity of CT. SCFAs promoted B cell Ab production by promoting dendritic cell production of BAFF and ALDH1a2, which induced B cell expression of IFN regulatory factor 4, Blimp1, and XBP1, the plasma B cell differentiation-related genes. Furthermore, when infected with Citrobacter rodentium, GPR43-/- mice exhibited decreased Ab responses and were more susceptible to infection, whereas the administration of SCFAs promoted intestinal Ab responses in wild-type mice. Our study thereby demonstrated a critical role of gut microbiota and their metabolite SCFAs in promoting mucosal adjuvant activity of CT through GPR43.
Copyright © 2019 by The American Association of Immunologists, Inc.
Conflict of interest statement
Figures




References
-
- Ranasinghe C 2014. New advances in mucosal vaccination. Immunol Lett 161: 204–206. - PubMed
-
- Lycke N 2012. Recent progress in mucosal vaccine development: potential and limitations. Nat Rev Immunol 12: 592–605. - PubMed
-
- Freytag LC, and Clements JD. 2005. Mucosal adjuvants. Vaccine 23: 1804–1813. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

