Direct-acting oral anticoagulants (DOACs) in pregnancy: new insight from VigiBase®
- PMID: 31076635
- PMCID: PMC6510783
- DOI: 10.1038/s41598-019-43715-4
Direct-acting oral anticoagulants (DOACs) in pregnancy: new insight from VigiBase®
Abstract
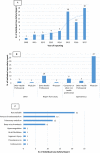
We aimed to perform an analysis of individual case safety reports retrieved after the Standardized MedDRA Query "Pregnancy and neonatal topics" for which Direct-Acting Oral Anticoagulants (DOACs) were claimed as suspected/interacting drugs. Additionally, to investigate if exists a disproportion of cases reporting "Pregnancy and neonatal topics" adverse events rather than other adverse events for DOACs in comparison with all other drugs registered in VigiBase or warfarin. VigiBase, the World Health Organization (WHO)'s global database of individual case safety reports was used as data source. Forty-two cases of abortion were detected of which 18 (42.8%) had alternative causes for its occurrence. Fourteen cases reported congenital anomaly (8 cases) or low birth weight baby/fetal growth restriction (6 cases) of which 62.5% and 33.3% had at least one confounder, respectively. In the disproportionality analyses, a potential safety signal for spontaneous abortion emerged for rivaroxaban (Reporting Odds Ratio, ROR 2.70; 95% CI 1.79-4.07) and apixaban (ROR 6.76; 95% CI 2.99-15.25). However, when the same analyses were performed using only cases without alternative causes, no statistically significant associations for rivaroxaban when compared to all other drugs (ROR 1.05; 95% CI 0.54-2.02) or warfarin (ROR 0.79; 95% CI 0.47-1.32) were found. For apixaban, we found a statistically significant ROR for induced abortion when compared to all other drugs or warfarin. For the majority of cases claiming DOACs-induced teratogenic effects, spontaneous or induced abortion there was at least one alternative cause explaining the occurrence of the adverse events. For rivaroxaban, when cases without confounders were considered, no safety signals emerged. However, for apixaban, we found a potential safety signal suggesting an increased probability of reporting spontaneous/induced abortion rather than other events when compared to all other drugs or warfarin.
Conflict of interest statement
Dr. Maurizio Sessa, Dr. Annamaria Mascolo, Dr. Torbjörn Callréus,Prof. Annalisa Capuano, and Prof. Francesco Rossi declare no competing interest that are directly relevant to the contents of this work. Prof. Morten Andersen has participated in research projects funded by AstraZeneca, H. Lundbeck & Mertz, Novartis, Pfizer and Janssen, with grants paid to the institutions where he has been employed, and has personally received fees from Medicademy, the Danish Pharmaceutical Industry Association, for leading and teaching pharmacoepidemiology courses.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

