Breaking Down Barriers: How Understanding Celiac Disease Pathogenesis Informed the Development of Novel Treatments
- PMID: 31076989
- PMCID: PMC6586517
- DOI: 10.1007/s10620-019-05646-y
Breaking Down Barriers: How Understanding Celiac Disease Pathogenesis Informed the Development of Novel Treatments
Abstract
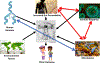
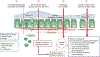
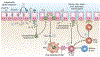
For decades, the pathogenesis of a variety of human diseases has been attributed to increased intestinal paracellular permeability even though scientific evidence supporting this hypothesis has been tenuous. Nevertheless, during the past decade, there have been a growing number of publications focused on human genetics, the gut microbiome, and proteomics, suggesting that loss of mucosal barrier function, particularly in the gastrointestinal tract, may substantially affect antigen trafficking, ultimately causing chronic inflammation, including autoimmunity, in genetically predisposed individuals. The gut mucosa works as a semipermeable barrier in that it permits nutrient absorption and also regulates immune surveillance while retaining potentially harmful microbes and environmental antigens within the intestinal lumen. Celiac disease (CD), a systemic, immune-mediated disorder triggered by gluten in genetically susceptible individuals, is associated with altered gut permeability. Pre-clinical and clinical studies have shown that gliadin, a prolamine component of gluten that is implicated in CD pathogenesis, is capable to disassembling intercellular junctional proteins by upregulating the zonulin pathway, which can be inhibited by the zonulin antagonist larazotide acetate. In this review, we will focus on CD as a paradigm of chronic inflammatory diseases in order to outline the contribution of gut paracellular permeability toward disease pathogenesis; moreover, we will summarize current evidence derived from available clinical trials of larazotide acetate in CD.
Keywords: Antigen trafficking; Autoimmunity; Gut permeability; Inflammation; Tight junctions; Zonulin.
Conflict of interest statement
Conflict of interest: Dr. Fasano is co-founder and stock holder of Alba Therapeutics, a company developing treatments complementary to the gluten free diet by exploiting gut permeability; Dr. Valitutti has no conflict of interest.
Figures
References
-
- Husby S Koletzko S, Korponay-Szabo IR et al. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition guidelines for the diagnosis of coeliac disease. J Pediatr Gastroenterol Nutr. 2012;54:136–60. - PubMed
-
- Catassi C, Kryszak D, Bhatti B et al. Natural history of celiac disease autoimmunity in a USA cohort followed since 1974. Ann Med. 2010; 42:530–538. - PubMed
-
- Lionetti E, Castellaneta S, Francavilla R et al. Introduction of gluten, HLA status, and the risk of celiac disease in children. N Engl J Med. 2014; 371:1295–303. - PubMed
-
- Fasano A Celiac disease - how to handle a clinical chameleon. N Engl J Med 2003; 348:2568–70 - PubMed
-
- Tapsas D, Hollén E, Stenhammar L et al. The clinical presentation of coeliac disease in 1030 Swedish children: Changing features over the past four decades. Dig Liver Dis 2016; 48:16–22. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

