Supervised Machine Learning with CITRUS for Single Cell Biomarker Discovery
- PMID: 31077114
- PMCID: PMC7458409
- DOI: 10.1007/978-1-4939-9454-0_20
Supervised Machine Learning with CITRUS for Single Cell Biomarker Discovery
Abstract
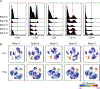
CITRUS is a supervised machine learning algorithm designed to analyze single cell data, identify cell populations, and identify changes in the frequencies or functional marker expression patterns of those populations that are significantly associated with an outcome. The algorithm is a black box that includes steps to cluster cell populations, characterize these populations, and identify the significant characteristics. This chapter describes how to optimize the use of CITRUS by combining it with upstream and downstream data analysis and visualization tools.
Keywords: Biomarker discovery; CITRUS; Supervised machine learning; viSNE.
Figures
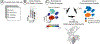


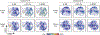

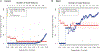
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

