Identification and characterisation of transient receptor potential melastatin 2 and CD38 channels on natural killer cells using the novel application of flow cytometry
- PMID: 31077146
- PMCID: PMC6509826
- DOI: 10.1186/s12865-019-0293-0
Identification and characterisation of transient receptor potential melastatin 2 and CD38 channels on natural killer cells using the novel application of flow cytometry
Abstract
Background: Natural Killer (NK) cells are effector lymphocytes of the innate immune system and are subclassed into CD56BrightCD16Dim/- and CD56DimCD16+ NK cells. Intracellular calcium (Ca2+) is fundamental to regulate a number of intracellular signalling pathways and functions in NK cells, which are essential in mediating their natural cytotoxic function. Transient receptor potential melastatin 2 (TRPM2) is a Ca2+-permeable non-selective cation channel that possesses a critical role in calcium-dependent cell signalling to maintain cellular homeostasis. TRPM2 and CD38 protein surface expression has yet to be determined on NK cells using flow cytometry. Characterisation of TRPM2 has been previously identified by in vivo models, primarily using methods such as genetic remodification, immunohistochemistry and whole cell electrophysiology. The aim of this study was to develop an in vitro methodology to characterise TRPM2 and CD38 surface expression on NK cell subsets using an antibody that has not been previously applied using flow cytometry.
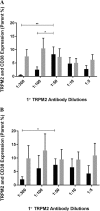
Results: At 2 h/1 h, TRPM2 (Fig. 2 A, B, p < 0.05) and TRPM2/CD38 (Fig. 3A, B, p < 0.05) surface expression significantly increased between 1:300 and 1:50 at 2 h/1 h. TRPM2/CD38 surface expression furthermore increased between 1:100 and 1:50 at 2 h/1 h (Fig. 3A, p < 0.05). Interestingly, TRPM2/CD38 surface expression significantly decreased from 1:50 to 1:5 on CD56BrightCD16Dim/- NK cells. These consistent findings highlight that 1:50 is the optimal antibody dilution and threshold to measure TRPM2 and TRPM2/CD38 surface expression on NK subsets. 2 h/1 h was determined as the optimal incubation period to ensure a sufficient timeframe for maximal antibody binding and surface expression.
Conclusion: For the first time, we describe an in vitro methodology to characterise TRPM2 and CD38 surface expression on NK cells in healthy participants. Finally, using an antibody that has not been previously applied in flow cytometry, we determined an antibody concentration and incubation time that is robust, rapid and sensitive for the application of flow cytometry.
Keywords: Antibody; Flow cytometry; Natural killer cells; Transient receptor potential Melastatin 2.
Conflict of interest statement
Ethics approval and consent to participate
All participants provided written consent and the study was approved by the Griffith University Human Research Ethics Committee (HREC/15/QGC/63).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

