AVAiLABLE NIS - AVASTIN® in lung cancer treatment in routine oncology practice in Germany
- PMID: 31077164
- PMCID: PMC6511164
- DOI: 10.1186/s12885-019-5618-0
AVAiLABLE NIS - AVASTIN® in lung cancer treatment in routine oncology practice in Germany
Abstract
Background: Bevacizumab (Avastin®), a recombinant humanized monoclonal antibody, in combination with platinum-doublet chemotherapy has become a routine treatment for advanced non-small-cell lung cancer (NSCLC). The post-authorization, non-interventional study 'AVAiLABLE' assessed the effectiveness and safety of bevacizumab combined with chemotherapy as first-line treatment.
Methods: Nine hundred and eighty-seven adult patients (mean age 61.5 years, 59.8% male) with non-resectable advanced, metastatic or recurrent, predominantly non-squamous NSCLC were evaluated at 185 sites across Germany. 72.8% of the patients had stage IV disease at start of observation, 90.1% had histologically confirmed adenocarcinoma and 80.8% met the bevacizumab label 'NSCLC other than predominantly squamous cell histology'. According to bevacizumab label, chemotherapy plus bevacizumab was recommended, followed by bevacizumab maintenance therapy. Effectiveness endpoints included response rates and progression-free survival (PFS); safety endpoints comprised adverse drug reactions (ADRs). Patients were followed until progression or intolerable toxicity. Data were evaluated by descriptive statistical methods.
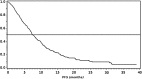
Results: Median PFS was 7.4 months (95% CI: 7.1; 8.4), overall response rate (ORR) 45.6% and disease control rate (DCR) 75%. The majority of patients (72.7%) achieved partial response or stable disease. Complete response was reached by 2.3%. 33.6% of patients experienced an ADR of grade ≥ 3. Bevacizumab-related ADRs of grade ≥ 3 occurred in 5.7% of patients, with the highest incidence for leukopenia, neutropenia, and hypertension.
Conclusions: Results of the non-interventional study 'AVAiLABLE' confirmed the effectiveness and safety of bevacizumab in combination with platinum-based chemotherapy as first-line treatment for advanced NSCLC in accordance with previous studies. No new safety signals were identified. Maintenance therapy with bevacizumab was well tolerated and safe even over extended periods (> 20 cycles).
Trial registration: ClinicalTrials.gov Identifier: NCT02596958; registered on 4 November 2015.
Keywords: Adenocarcinoma; Advanced non-small-cell lung cancer; Bevacizumab (Avastin®) plus chemotherapy; Post-authorization study.
Conflict of interest statement
Ethics approval and consent to participate
The study was evaluated by the Ethics Committee of the Medical Association of Lower Saxony (‘Ärztekammer Niedersachsen’) in Hannover (Germany) prior to start of the observation and performed in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. All patients gave signed, informed consent to participate in the study.
Consent for publication
Not applicable.
Competing interests
ZMO, LD, LC, GC, MH, THW have nothing to disclose. VEG reports other from Roche Pharma AG during the conduct of the study.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Gesundheitsberichterstattung des Bundes: Bericht zum Krebsgeschehen in Deutschland 2016; Berlin, November 2016. http://www.krebsdaten.de/Krebs/DE/Content/Publikationen/Krebsgeschehen/K.... Accessed 06 February 2019.
-
- Noone AM, Howlader N, Krapcho M, Miller D, Brest A, Yu M, et al., (editors). SEER Cancer Statistics Review, 1975–2015, National Cancer Institute. Bethesda, MD. https://seer.cancer.gov/csr/1975_2015/, based on November 2017 SEER data submission, posted to the SEER web site, 2018. Accessed 06 February 2019.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous