Somatostatin in renal physiology and autosomal dominant polycystic kidney disease
- PMID: 31077332
- PMCID: PMC7462725
- DOI: 10.1093/ndt/gfz054
Somatostatin in renal physiology and autosomal dominant polycystic kidney disease
Abstract
Autosomal dominant polycystic kidney disease (ADPKD) is characterized by progressive cyst formation, leading to growth in kidney volume and renal function decline. Although therapies have emerged, there is still an important unmet need for slowing the rate of disease progression in ADPKD. High intracellular levels of adenosine 3',5'-cyclic monophosphate (cAMP) are involved in cell proliferation and fluid secretion, resulting in cyst formation. Somatostatin (SST), a hormone that is involved in many cell processes, has the ability to inhibit intracellular cAMP production. However, SST itself has limited therapeutic potential since it is rapidly eliminated in vivo. Therefore analogues have been synthesized, which have a longer half-life and may be promising agents in the treatment of ADPKD. This review provides an overview of the complex physiological effects of SST, in particular renal, and the potential therapeutic role of SST analogues in ADPKD.
Keywords: ADPKD; cAMP; renal physiology; somatostatin; somatostatin analogues.
© The Author(s) 2019. Published by Oxford University Press on behalf of ERA-EDTA.
Figures


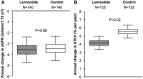
References
-
- Neumann HP, Jilg C, Bacher J. et al. Epidemiology of autosomal-dominant polycystic kidney disease: an in-depth clinical study for south-western Germany. Nephrol Dial Transplant 2013; 28: 1472–1487 - PubMed
-
- Bae KT, Zhu F, Chapman AB. et al. Magnetic resonance imaging evaluation of hepatic cysts in early autosomal-dominant polycystic kidney disease: the Consortium for Radiologic Imaging Studies of Polycystic Kidney Disease cohort. Clin J Am Soc Nephrol 2005; 1: 64–69 - PubMed
-
- van Keimpema L, Nevens F, Adam R. et al. Excellent survival after liver transplantation for isolated polycystic liver disease: an European Liver Transplant Registry study. Transpl Int 2011; 24: 1239–1245 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

