3D Bioprinting of cardiac tissue and cardiac stem cell therapy
- PMID: 31078513
- PMCID: PMC6702075
- DOI: 10.1016/j.trsl.2019.04.004
3D Bioprinting of cardiac tissue and cardiac stem cell therapy
Abstract
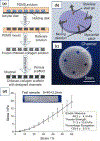
Cardiovascular tissue engineering endeavors to repair or regenerate damaged or ineffective blood vessels, heart valves, and cardiac muscle. Current strategies that aim to accomplish such a feat include the differentiation of multipotent or pluripotent stem cells on appropriately designed biomaterial scaffolds that promote the development of mature and functional cardiac tissue. The advent of additive manufacturing 3D bioprinting technology further advances the field by allowing heterogenous cell types, biomaterials, and signaling factors to be deposited in precisely organized geometries similar to those found in their native counterparts. Bioprinting techniques to fabricate cardiac tissue in vitro include extrusion, inkjet, laser-assisted, and stereolithography with bioinks that are either synthetic or naturally-derived. The article further discusses the current practices for postfabrication conditioning of 3D engineered constructs for effective tissue development and stability, then concludes with prospective points of interest for engineering cardiac tissues in vitro. Cardiovascular three-dimensional bioprinting has the potential to be translated into the clinical setting and can further serve to model and understand biological principles that are at the root of cardiovascular disease in the laboratory.
Copyright © 2019 Elsevier Inc. All rights reserved.
Figures





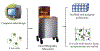

References
-
- Organization, W.H., Global Health Estimates 2016: Deaths by Cause, Age, Sex, by Country and by Region. 2018: Geneva.
-
- Duan B, State-of-the-Art Review of 3D Bioprinting for Cardiovascular Tissue Engineering. Annals of Biomedical Engineering, 2017. 45(1): p. 195–209. - PubMed
-
- Mario Gaudino UB, Stephen Fremes, et al., Radial-Artery or Saphenous-Vein Grafts in Coronary-Artery Bypass Surgery. The New England Journal of Medicine, 2018. 378(22): p. 2069–2077. - PubMed
-
- Manji Rizwan A., Burcin Ekser AHM, et al., Porcine bioprosthetic heart valves: The next generation. American Heart Journal, 2012. 164(2): p. 177–185. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

