A Ferret Model of Encephalopathy of Prematurity
- PMID: 31079096
- PMCID: PMC6658350
- DOI: 10.1159/000498968
A Ferret Model of Encephalopathy of Prematurity
Abstract
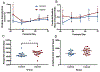
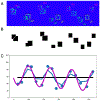
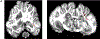
There is an ongoing need for relevant animal models in which to test therapeutic interventions for infants with neurological sequelae of prematurity. The ferret is an attractive model species as it has a gyrified brain with a white-to-gray matter ratio similar to that in the human brain. A model of encephalopathy of prematurity was developed in postnatal day 10 (P10) ferret kits, considered to be developmentally equivalent to infants of 24-26 weeks' gestation. Cross-fostered P10 ferret kits received 5 mg/kg of lipopolysaccharide (LPS) before undergoing consecutive hypoxia-hyperoxia-hypoxia (60 min at 9%, 120 min at 60%, and 30 min at 9%). Control animals received saline vehicle followed by normoxia. The development of basic reflexes (negative geotaxis, cliff aversion, and righting) as well as gait coordination on an automated catwalk were assessed between P28 and P70, followed by ex vivo magnetic resonance imaging (MRI) and immunohistochemical analysis. Compared to controls, injured animals had slower overall reflex development between P28 and P40, as well as smaller hind-paw areas consistent with "toe walking" at P42. Injured animals also displayed significantly greater lateral movement during CatWalk assessment as a result of reduced gait coordination. Ex vivo MRI showed widespread white-matter hyperintensity on T2-weighted imaging as well as altered connectivity patterns. This coincided with white-matter dysmaturation characterized by increased intensity of myelin basic protein staining, white-matter thinning, and loss of oligodendrocyte transcription factor 2 (OLIG2)-positive cells. These results suggest both pathological and motor deficits consistent with premature white-matter injury. This newborn ferret model can therefore provide an additional platform to assess potential therapies before translation to human clinical trials.
Keywords: Brain; Development; Neonatal brain injury; Prematurity; White-matter injury.
© 2019 S. Karger AG, Basel.
Figures









References
-
- Martin JA, Hamilton BE, Osterman MJK, et al. Births: Final Data for 2016. National vital statistics reports : from the Centers for Disease Control and Prevention, National Center for Health Statistics, National Vital Statistics System. 2018;67(1):1–55. - PubMed
-
- Johnson S, Marlow N. Early and long-term outcome of infants born extremely preterm. Archives of Disease in Childhood. 2017;102(1):97. - PubMed
-
- Reich B, Hoeber D, Bendix I, Felderhoff-Mueser U. Hyperoxia and the Immature Brain. Developmental neuroscience. 2016;38(5):311–30. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

