Bi-allelic Variants in DYNC1I2 Cause Syndromic Microcephaly with Intellectual Disability, Cerebral Malformations, and Dysmorphic Facial Features
- PMID: 31079899
- PMCID: PMC6556908
- DOI: 10.1016/j.ajhg.2019.04.002
Bi-allelic Variants in DYNC1I2 Cause Syndromic Microcephaly with Intellectual Disability, Cerebral Malformations, and Dysmorphic Facial Features
Abstract
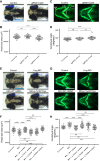
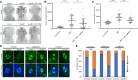
Cargo transport along the cytoplasmic microtubular network is essential for neuronal function, and cytoplasmic dynein-1 is an established molecular motor that is critical for neurogenesis and homeostasis. We performed whole-exome sequencing, homozygosity mapping, and chromosomal microarray studies in five individuals from three independent pedigrees and identified likely-pathogenic variants in DYNC1I2 (Dynein Cytoplasmic 1 Intermediate Chain 2), encoding a component of the cytoplasmic dynein 1 complex. In a consanguineous Pakistani family with three affected individuals presenting with microcephaly, severe intellectual disability, simplification of cerebral gyration, corpus callosum hypoplasia, and dysmorphic facial features, we identified a homozygous splice donor site variant (GenBank: NM_001378.2:c.607+1G>A). We report two additional individuals who have similar neurodevelopmental deficits and craniofacial features and harbor deleterious variants; one individual bears a c.740A>G (p.Tyr247Cys) change in trans with a 374 kb deletion encompassing DYNC1I2, and an unrelated individual harbors the compound-heterozygous variants c.868C>T (p.Gln290∗) and c.740A>G (p.Tyr247Cys). Zebrafish larvae subjected to CRISPR-Cas9 gene disruption or transient suppression of dync1i2a displayed significantly altered craniofacial patterning with concomitant reduction in head size. We monitored cell death and cell cycle progression in dync1i2a zebrafish models and observed significantly increased apoptosis, likely due to prolonged mitosis caused by abnormal spindle morphology, and this finding offers initial insights into the cellular basis of microcephaly. Additionally, complementation studies in zebrafish demonstrate that p.Tyr247Cys attenuates gene function, consistent with protein structural analysis. Our genetic and functional data indicate that DYNC1I2 dysfunction probably causes an autosomal-recessive microcephaly syndrome and highlight further the critical roles of the dynein-1 complex in neurodevelopment.
Keywords: DYNC1I2; apoptosis; autosomal recessive; developmental delay; dynein; dysmorphic facial features; intellectual disability; microcephaly; mitotic spindle; zebrafish.
Copyright © 2019 American Society of Human Genetics. Published by Elsevier Inc. All rights reserved.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

